Mitomycin C from Streptomyces caespitosus
meets USP testing specifications
Manufacturer: Sigma Aldrich
CAS Number: 50-07-7
Synonym(S): Mitomycin
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 MG | M0440-5-MG | In Stock | ₹ 9,807.45 |
| 25 MG | M0440-25-MG | In Stock | ₹ 47,575.88 |
M0440 - 5 MG
In Stock
Quantity
1
Base Price: ₹ 9,807.45
GST (18%): ₹ 1,765.341
Total Price: ₹ 11,572.791
biological source
Streptomyces caespitosus
Quality Level
200
color
blue-violet
solubility
H2O: 40 mg/mL, clear to very slightly hazy, colorlessH2O: soluble
antibiotic activity spectrum
Gram-negative bacteriaGram-positive bacteria
Mode of action
DNA synthesis | interferes
storage temp.
2-8°C
SMILES string
[H][C@]12CN3C4=C([C@@H](COC(N)=O)[C@@]3(OC)[C@@]1([H])N2)C(=O)C(N)=C(C)C4=O
InChI
1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1
InChI key
NWIBSHFKIJFRCO-WUDYKRTCSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Mitomycin | Sigma Aldrich | ₹ 1,25,342.68 | |
 | Mitomycin | Sigma Aldrich | ₹ 20,567.50 | |
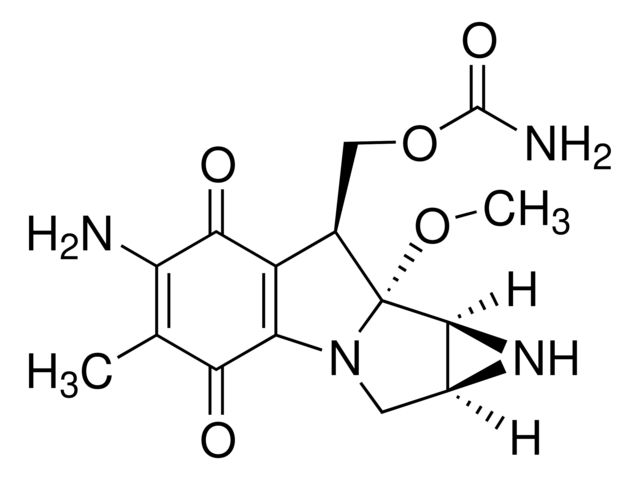 | Mitomycin C from Streptomyces caespitosus | Sigma Aldrich | ₹ 17,612.28 - ₹ 78,730.23 | |
 | Mitomycin C | Sigma Aldrich | ₹ 8,248.65 | |
 | Mitomycin C from Streptomyces caespitosus | Sigma Aldrich | ₹ 12,557.00 | |
 | Mitomycin C from Streptomyces caespitosus | Sigma Aldrich | ₹ 17,135.98 - ₹ 2,61,553.65 |
Description
- General description: Chemical structure: aziridine
- Application: Mitomycin C is produced by a strain of actinomyces, Streptomyces caespitosus. It contains three anticancer moieties, quinine, urethane, and aziridine groups. It is used to generate mitotically inactive feeder cells in cell culture systems, such as the mitotically inactive fibroblasts used in embryonic stem cell systems.
- Biochem/physiol Actions: Mode of Action: This product is an alkylating agent that specifically targets the guanine nucleoside sequence 5′-CpG-3′. It inhibits DNA synthesis by covalently reacting with DNA, forming crosslinks between complementary strands of DNA. This interaction prevents separation of complementary DNA strands, inhibiting DNA replication.Antimicrobial Spectrum: Mitomycin C has strong antitumor activity, especially against Ehrlich ascites tumor cells, and strong bactericidal action against gram-positive and gram-negative bacteria.
- Caution: Stock solutions should be filter sterilized and stored at 2-8 °C in the dark. Solutions at pH 6-9 can be stored at 0-5 °C for up to a week, but if a precipitate forms, a fresh solution should be prepared - the precipitated solution has been proven toxic to cells.
- Preparation Note: Mitomycin C is soluble in water at .5 mg/mL, with a pH of 6.0-7.5. It undergoes rapid degradation in acidic solutions with pH<6, and is mostly likely to retain activity in solutions with a pH between 6-9.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Carc. 2
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves