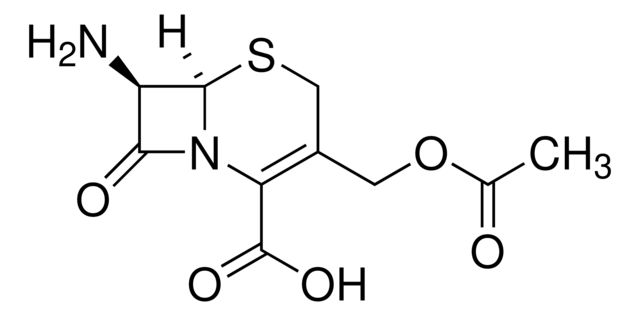
191140
7-Aminocephalosporanic acid
98%
Manufacturer: Sigma Aldrich
CAS Number: 957-68-6
Synonym(S): 7-ACA
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 G | 191140-5-G | In Stock | ₹ 8,324.43 |
191140 - 5 G
In Stock
Quantity
1
Base Price: ₹ 8,324.43
GST (18%): ₹ 1,498.397
Total Price: ₹ 9,822.827
Quality Level
200
Assay
98%
form
powder
optical activity
[α]19/D +90°, c = 0.5 in KH2PO4/trace NaOH
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
antibiotic activity spectrum
Gram-positive bacteria
application(s)
peptide synthesis
Mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Cefazolin Impurity H | Supelco | ₹ 54,622.95 | |
 | (6R,7R)-3-(Acetoxymethyl)-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Cefoperazone Impurity | ChemScene | ₹ 1,691.00 - ₹ 16,376.00 | |
 | 7-Aminocephalosporanic acid | ChemScene | ₹ 1,691.00 - ₹ 22,339.00 | |
 | 957-68-6 | (6R,7R)-3-[(acetyloxy)methyl]-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | A2B Chem | ₹ 534.00 - ₹ 11,659.00 |
Description
- General description: Chemical structure: ß-lactam
- Application: 7-Aminocephalosporanic acid (7-ACA) can be used as a starting material to synthesize: Cefotaxime by acylation reaction with S-benzothiazol-2-yl(2-amino-4-thiazolyl)(methoxyimino)thioacetate (MAEM) in the presence of triethylamine.[1]Cefpodoxime proxetil, a third-generation antibiotic via cefotaxime intermediate.[1]Sodium 3′-substituted cephalosporanate sulfone derivatives as potential inhibitors of β-lactamase.[2]
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
Quality Level: 200
Assay: 98%
form: powder
optical activity: [α]19/D +90°, c = 0.5 in KH2PO4/trace NaOH
reaction suitability: reaction type: solution phase peptide synthesis
mp: >300 °C (lit.)
antibiotic activity spectrum: Gram-positive bacteria
application(s): peptide synthesis
Mode of action: cell wall synthesis | interferes
storage temp.: 2-8°C
Quality Level:
200
Assay:
98%
form:
powder
optical activity:
[α]19/D +90°, c = 0.5 in KH2PO4/trace NaOH
reaction suitability:
reaction type: solution phase peptide synthesis
mp:
>300 °C (lit.)
antibiotic activity spectrum:
Gram-positive bacteria
application(s):
peptide synthesis
Mode of action:
cell wall synthesis | interferes
storage temp.:
2-8°C