1A00560
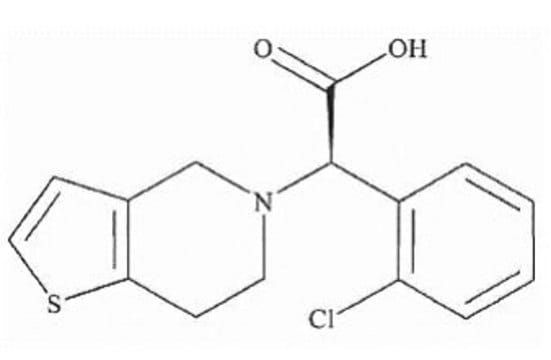
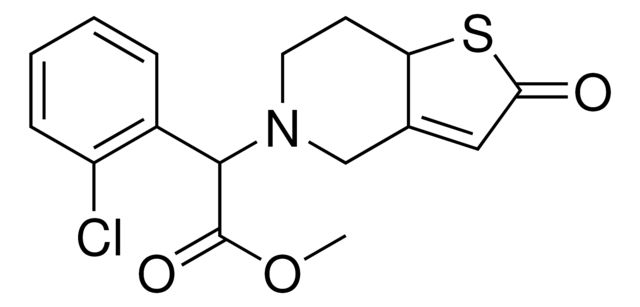
2-Oxo Clopidogrel (Mixture Of Diastereomers)
Pharmaceutical Analytical Impurity (PAI)
Manufacturer: Sigma Aldrich
CAS Number: 109904-27-0
Synonym(S): (methyl 2-(2-chlorophenyl)-2-(2-oxo-2,6,7,7a-tetrahydrothieno[3,2-c]pyridin-5(4H)-yl)acetate)
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 MG | 1A00560-10-MG | In Stock | ₹ 68,976.90 |
1A00560 - 10 MG
In Stock
Quantity
1
Base Price: ₹ 68,976.90
GST (18%): ₹ 12,415.842
Total Price: ₹ 81,392.742
grade
pharmaceutical analytical impurity (PAI)
Agency
USP
manufacturer/tradename
USP
application(s)
pharmaceutical
format
neat
storage temp.
2-8°C
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
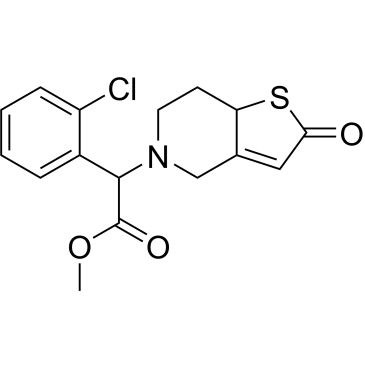 | (±)-2-Oxo clopidogrel | ChemScene | ₹ 76,540.00 | |
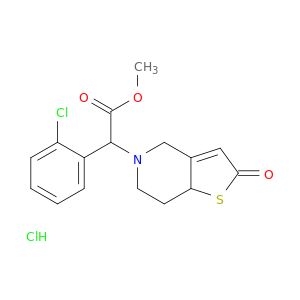 | 109904-27-0 | 2-Oxo Clopidogrel Hydrochloride(Mixture of Diastereomers) | A2B Chem | ₹ 7,387.00 - ₹ 28,569.00 |
Description
- General description: 2-Oxo Clopidogrel (Mixture Of Diastereomers) is a USP Pharmaceutical Analytical Impurity (PAI). USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.Associated Drug Substance: Clopidogrel BisulfateTherapeutic Area: Cardiovascular.For more information about this PAI, visit here.
- Application: 2-Oxo Clopidogrel (Mixture Of Diastereomers) (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
- Features and Benefits: USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:1. Conduct analytical tests during early formulation feasibility studies.2. Determine degradation impurities produced during stress studies.3. Develop, validate, and transfer analytical methods.4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.5. Record retention times and/or spectra6. Determine relative response factors.7. Identify unknown impurities that formed during ICH stability conditions.8. Identify impurities that are present in the Reference Listed Drug9. Test for and profile impurities not listed in drug substance and drug product monographs.
- Analysis Note: These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
- Other Notes: Sales restrictions may apply.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P260 - P273 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338
Hazard Classifications
Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable