907294
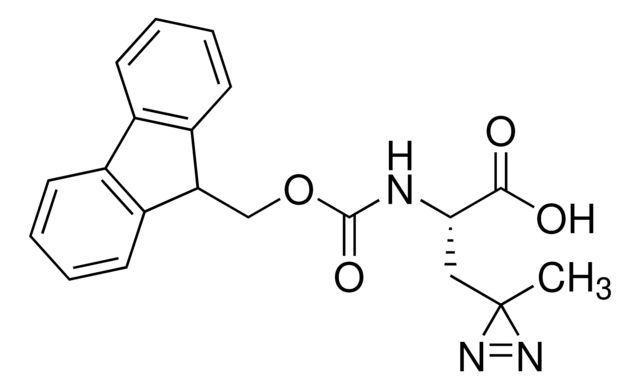
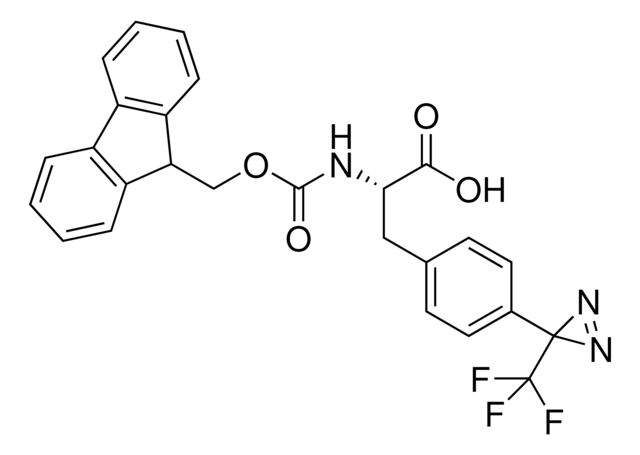
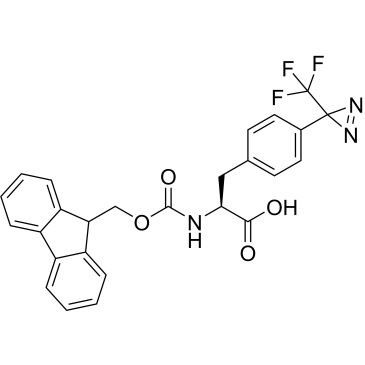
Fmoc-L-Photo-Phe-OH
≥95%
Manufacturer: Sigma Aldrich
CAS Number: 133342-64-0
Synonym(S): (S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, N-α-(9-Fluorenylmethyloxycarbonyl)-4-(trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, Fmoc-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 50 MG | 907294-50-MG | In Stock | ₹ 55,802.88 |
907294 - 50 MG
In Stock
Quantity
1
Base Price: ₹ 55,802.88
GST (18%): ₹ 10,044.518
Total Price: ₹ 65,847.398
Assay
≥95%
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
−20°C
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Fmoc-L-Photo-Phe-OH | ChemScene | ₹ 1,65,629.00 |
Description
- Application: Fmoc-L-Photo-Phe-OH is a diazirine-containing, Fmoc-protected phenylalanine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907340.Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
- Other Notes: Covalent modifier-type aggregation inhibitor of amyloid-β based on a cyclo-KLVFF motifMode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass SpectrometryTrifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formationSimple and Versatile Method for Tagging Phenyldiazirine PhotophoresFishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe SynthesisPhoto-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
SAFETY INFORMATION
WGK
WGK 3