A3611
Arachidonic acid
from non-animal source, ≥98.5% (GC)
Manufacturer: Sigma Aldrich
CAS Number: 506-32-1
Synonym(S): cis,cis,cis,cis-5,8,11,14-Eicosatetraenoic acid, Eicosa-5Z,8Z,11Z,14Z-tetraenoic acid, Immunocytophyte
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 MG | A3611-10-MG | In Stock | ₹ 9,341.98 |
| 100 MG | A3611-100-MG | In Stock | ₹ 17,460.73 |
| 1 G | A3611-1-G | In Stock | ₹ 95,422.38 |
A3611 - 10 MG
In Stock
Quantity
1
Base Price: ₹ 9,341.98
GST (18%): ₹ 1,681.556
Total Price: ₹ 11,023.536
biological source
non-animal source
Quality Level
300
Assay
≥98.5% (GC)
form
liquid
refractive index
n20/D 1.4872 (lit.)
bp
169-171 °C/0.15 mmHg (lit.)
mp
−49 °C (lit.)
density
0.922 g/mL at 25 °C (lit.)
functional group
carboxylic acid
lipid type
omega FAs
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Arachidonic acid, ≥97.0% (GC), MilliporeSigma™ Supelco™ | MilliporeSigma Supelco | ₹ 24,430.50 | |
 | Sigma Aldrich Fine Chemicals Biosciences Arachidonic acid | 506-32-1 | MFCD00004417 | 10mg | Sigma Aldrich Fine Chemicals Biosciences | ₹ 10,073.91 | |
 | eMolecules Medchem Express / Arachidonic acid / 5mg / 437899075 / HY-109590 / / 506-32-1 / MFCD00004417 / 304.474 / C20H32O2 | eMolecules | ₹ 6,550.40 | |
 | Cayman Chemical Arachidonic Acid (peroxide free), 506-32-1, 25 mg | Cayman Chemical | ₹ 4,236.40 | |
 | Cayman Chemical Arachidonic Acid, 506-32-1, 50 mg | Cayman Chemical | ₹ 3,666.80 | |
 | Nu Chek Prep Inc Arachidonic Acid | 506-32-1 | 506-32-1 | 500mg | Nu Chek Prep Inc | ₹ 9,267.57 | |
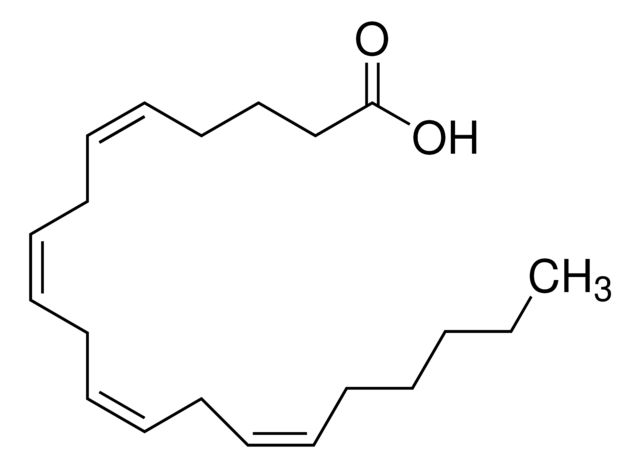 | Arachidonic acid | Supelco | ₹ 19,095.30 | |
 | Arachidonic acid | Supelco | ₹ 24,099.60 | |
 | Arachidonic acid | Sigma Aldrich | ₹ 23,187.15 - ₹ 44,566.53 | |
 | Arachidonic acid | Supelco | ₹ 19,095.30 |
Related Products
Description
- Application: <ul><li><strong>Molecular Mechanisms Associated with the Inhibitory Role of Long Chain n-3 PUFA in Colorectal Cancer:</strong> This study discusses the effects of long-chain polyunsaturated fatty acids, like arachidonic acid, on colorectal cancer mechanisms. The research focuses on the anti-inflammatory and cancer inhibitory roles through the modulation of lipid metabolism and signal transduction pathways (Jayathilake et al., 2024).</li><li><strong>Zhilining Formula alleviates DSS-induced colitis through suppressing inflammation and gut barrier dysfunction via the AHR/NF-Bp65 axis:</strong> This article presents arachidonic acid′s role in the suppression of inflammation and restoration of gut barrier function, crucial for understanding inflammatory diseases and developing therapeutic strategies (Zhou et al., 2024).</li><li><strong>5,6-diHETE lactone (EPA-L) mediates hypertensive microvascular dilation by activating the endothelial GPR-PLC-IP(3) signaling pathway:</strong> Explores the cardiovascular implications of arachidonic acid metabolites, specifically their role in microvascular responses, which could influence hypertension treatment strategies (Asulin et al., 2024).</li></ul>
- Biochem/physiol Actions: Arachidonic acid (AA) is an unsaturated ω6 fatty acid constituent of the phospholipids of cell membranes. Phospholipase A2 releases AA from the membrane phospholipids in response to inflammation. AA is subsequently metabolized to prostaglandins and thromboxanes by at least two cyclooxygenase (COX) isoforms, to leukotrienes and lipoxins by lipoxygenases, and to epoxyeicosatrienoic acids via cytochrome p450-catalyzed metabolism. AA and its metabolites play important roles in a variety of biological processes, including signal transduction, smooth muscle contraction, chemotaxis, cell proliferation and differentiation, and apoptosis. AA has been demonstrated to bind to the a subunit of G protein and inhibit the activity of Ras GTPase-activating proteins (GAPs). Cellular uptake of AA is energy dependent and involves protein-facilitated transport across the plasma membrane.
- Packaging: Sealed ampule
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P264 - P280 - P302 + P352 - P305 + P351 + P338 - P332 + P313 - P337 + P313
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
WGK
WGK 3
Flash Point(F)
235.4 °F
Flash Point(C)
113 °C