431966
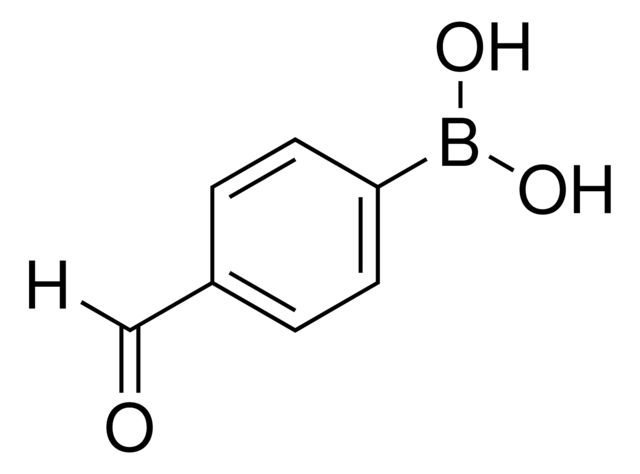
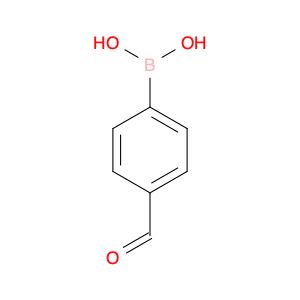
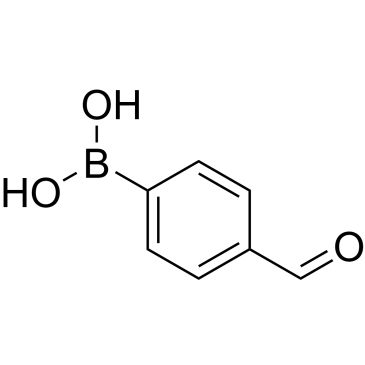
4-Formylphenylboronic acid
≥95.0%
Manufacturer: Sigma Aldrich
CAS Number: 87199-17-5
Synonym(S): 4-(Dihydroxyboryl)benzaldehyde, 4-Boronobenzaldehyde, 4-Formylbenzeneboronic acid, p-Formylbenzeneboronic acid, p-Formylphenylboronic acid
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 431966-1-G | In Stock | ₹ 1,720.00 |
| 5 G | 431966-5-G | In Stock | ₹ 9,212.08 |
| 25 G | 431966-25-G | In Stock | ₹ 21,370.00 |
431966 - 1 G
In Stock
Quantity
1
Base Price: ₹ 1,720.00
GST (18%): ₹ 309.60
Total Price: ₹ 2,029.60
Quality Level
100
Assay
≥95.0%
mp
237-242 °C (lit.)
SMILES string
OB(O)c1ccc(C=O)cc1
InChI
1S/C7H7BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5,10-11H
InChI key
VXWBQOJISHAKKM-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | eMolecules Ambeed / 4-Formylphenylboronic acid / 10g / 552711481 / A340775 / / 87199-17-5 / MFCD00151823 / 149.940 / C7H7BO3 | eMolecules | ₹ 2,604.14 | |
 | Sigma Aldrich Fine Chemicals Biosciences 4-Formylphenylboronic acid >=95.0% | 87199-17-5 | MFCD00151823 | 25G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 34,125.27 | |
 | Sigma Aldrich Fine Chemicals Biosciences 4-Formylphenylboronic acid >=95.0% | 87199-17-5 | MFCD00151823 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 4,850.50 | |
 | Sigma Aldrich Fine Chemicals Biosciences 4-Formylphenylboronic acid >=95.0% | 87199-17-5 | MFCD00151823 | 5G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 13,192.47 | |
 | 4-Formylphenylboronic acid | Aaron Chemicals LLC | ₹ 356.00 - ₹ 21,004.00 | |
 | 4-Formylphenylboronic acid | ChemScene | ₹ 801.00 - ₹ 46,013.00 |
Related Products
Description
- Application: 4-Formylphenylboronic acid is a substrate for Suzuki cross-coupling reactions[1][2] and it can be used as a reagent for:Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.[3] Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.[4] Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.[5] Triethylamine-catalyzed three-component Hantzsch condensations.[6] Copper-catalyzed nitrations.[7] Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.[8] Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.[9] Palladium-catalyzed aerobic oxidative cross-coupling reactions.[10] The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.[11]The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.[12]The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.[13]A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
- Other Notes: Contains varying amounts of anhydride
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P261 - P272 - P280 - P302 + P352 - P333 + P313 - P362 + P364
Hazard Classifications
Skin Sens. 1
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves