Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate
Manufacturer: Sigma Aldrich
CAS Number: 79060-88-1
Synonym(S): Benzene, 1,3-bis(trifluoromethyl)-, boron complex, NaBARF, Tetrakis[3,5-bis(trifluoromethyl)phenyl]boron sodium
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 250 MG | 692360-250-MG | In Stock | ₹ 17,092.68 |
| 1 G | 692360-1-G | In Stock | ₹ 33,092.03 |
692360 - 250 MG
In Stock
Quantity
1
Base Price: ₹ 17,092.68
GST (18%): ₹ 3,076.682
Total Price: ₹ 20,169.362
form
powder
Quality Level
100
reaction suitability
core: sodiumreagent type: catalyst
SMILES string
[Na+].FC(F)(F)c1cc(cc(c1)C(F)(F)F)[B-](c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(c3cc(cc(c3)C(F)(F)F)C(F)(F)F)c4cc(cc(c4)C(F)(F)F)C(F)(F)F
InChI
1S/C32H12BF24.Na/c34-25(35,36)13-1-14(26(37,38)39)6-21(5-13)33(22-7-15(27(40,41)42)2-16(8-22)28(43,44)45,23-9-17(29(46,47)48)3-18(10-23)30(49,50)51)24-11-19(31(52,53)54)4-20(12-24)32(55,56)57;/h1-12H;/q-1;+1
InChI key
LTGMONZOZHXAHO-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Chemscene AbaChemscene,Sodium tetrakis(3,5-bis(t,79060-88-1,Formula:C32H12BF24Na,M. Wt. 886.20,>98% | Chemscene | ₹ 3,956.94 | |
 | eMolecules Ambeed / Sodium tetrakis(35-bis(trifluoromethyl)phenyl)borate / 1g / 525044238 / A136880 / / 79060-88-1 / MFCD00043323 / 886.210 / C32H12BF24Na | eMolecules | ₹ 2,969.04 | |
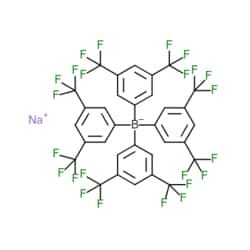 | Matrix Scientific Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]-borate, 79060-88-1, MFCD00043323, 1g | Matrix Scientific | ₹ 7,369.20 | |
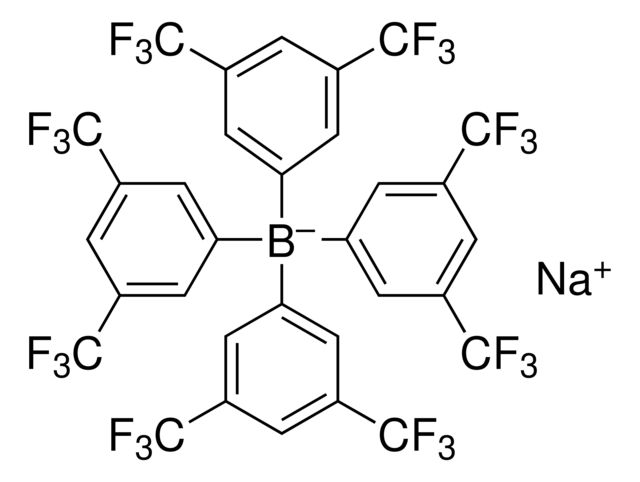 | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate | Supelco | ₹ 8,551.75 - ₹ 1,76,858.85 | |
 | Sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate | Aaron Chemicals LLC | ₹ 356.00 - ₹ 1,35,814.00 | |
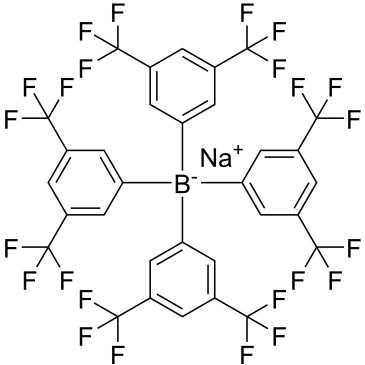 | Sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate | ChemScene | ₹ 1,335.00 - ₹ 1,44,892.00 |
Related Products
Description
- General description: Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, also known as NaBArF, is widely used as a catalyst in the cyclopolymerization of functionalized trienes.[1] NaBArF also acts as a precursor to synthesize other tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (TFPB)-based reagents.[2][3]Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, also known as NaBAr′4, is a stable and highly soluble compound that has been used as a source of the [BArF] counterion in chemical synthesis. It has been found to be resistant to degradation by various oxidants and highly lipophilic. This compound has been used as a convenient reagent for the generation and stabilization of cationic electrophilic metal alkyl complexes. It has also been used in the living polymerization of ethylene, oligomerization of α-olefins, and olefin hydrogenation and hydrosilylation.
- Application: The TFPB anion can be used to catalyze:In-situ diazo-coupling and N- and C-nitrosations.[4]Synthesis of oxirane from different carbonyl substrates and trimethylsulfonium chloride via phase-transfer catalysis.[5]Synthesis of ion selective membranes[6]9
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable