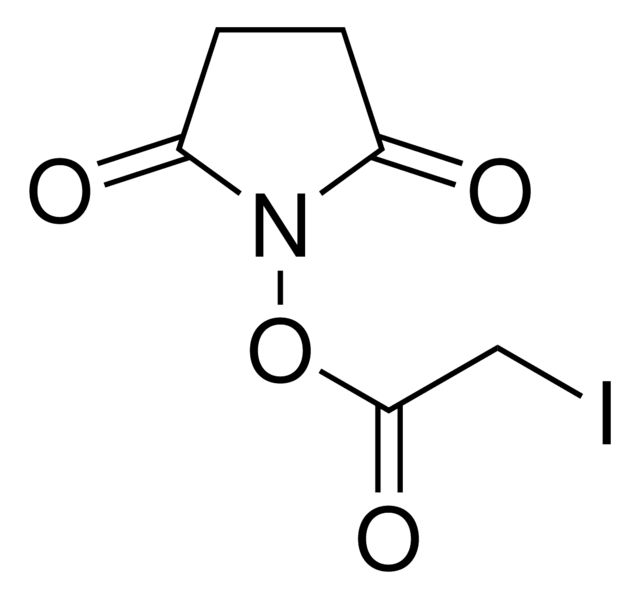
B81255
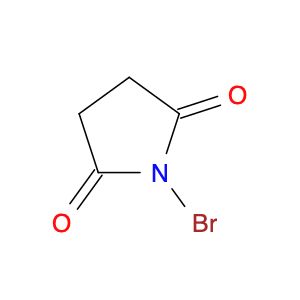
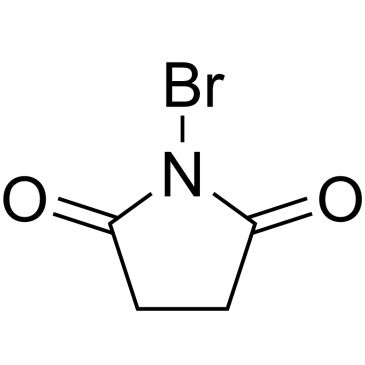
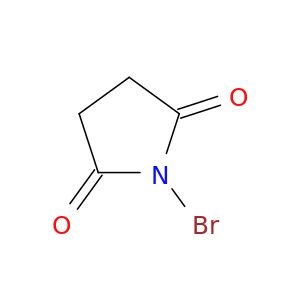
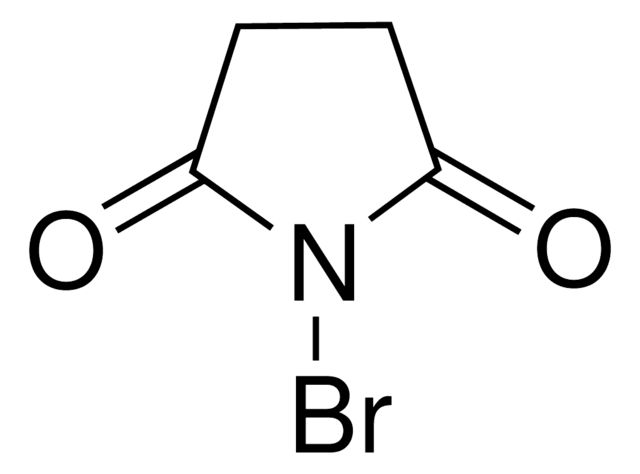
N-Bromosuccinimide
99%, for peptide synthesis, ReagentPlus®
Manufacturer: Sigma Aldrich
CAS Number: 128-08-5
Synonym(S): NBS
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 G | B81255-5-G | In Stock | ₹ 3,990.00 |
| 100 G | B81255-100-G | In Stock | ₹ 5,430.00 |
| 250 G | B81255-250-G | In Stock | ₹ 8,390.00 |
| 500 G | B81255-500-G | In Stock | ₹ 15,940.00 |
| 1 KG | B81255-1-KG | In Stock | ₹ 24,270.00 |
| 5 KG | B81255-5-KG | In Stock | ₹ 50,120.00 |
B81255 - 5 G
In Stock
Quantity
1
Base Price: ₹ 3,990.00
GST (18%): ₹ 718.20
Total Price: ₹ 4,708.20
product name
N-Bromosuccinimide, ReagentPlus®, 99%
Quality Level
200
product line
ReagentPlus®
Assay
99%
form
powder
mp
175-180 °C (dec.) (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
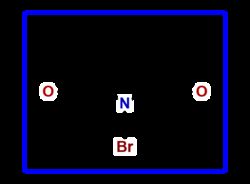
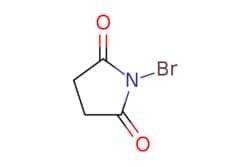
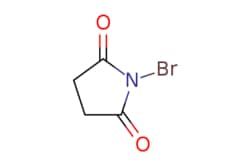
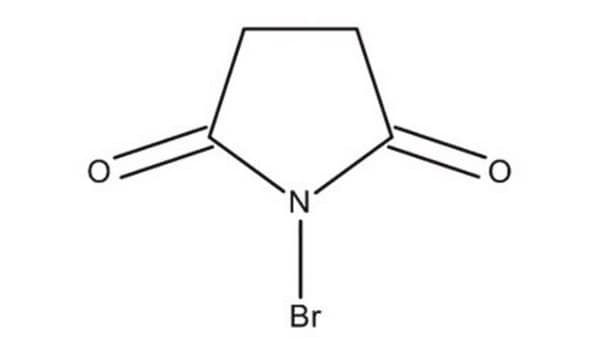
BrN1C(=O)CCC1=O
InChI
1S/C4H4BrNO2/c5-6-3(7)1-2-4(6)8/h1-2H2
Other Options
Description
- General description: N-Bromosuccinimide (NBS) is an organic compound commonly used as a brominating agent in organic synthesis. It is a convenient source of bromine radicals. It is used in radical bromination of allylic and benzylic positions. Additionally, NBS is also used as a reagent in electrophilic addition and electrophilic substitution reactions in organic chemistry.
- Application: N-Bromosuccinimide can be used as a reagent: In the Wohl-Ziegler reaction (bromination at allylic positions via a radical pathway). To synthesize benzils and aliphatic 1,2-diketones from hydrobenzoins and 1,2-diols in the presence of CCl4 as a solvent. To prepare tricyclic azepino[4,5-b]indoles from indole-β-enaminoesters or β-enaminones via Pictet–Spengler cyclization.To synthesize acylsilanes via oxidative hydrolysis of 2-silyl-1,3-dithianes.
- Features and Benefits: NBS is an easier and safer brominating agent to handle than bromine.
- Legal Information: ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P210 - P273 - P280 - P302 + P352 - P305 + P351 + P338 - P308 + P313
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2 - Met. Corr. 1 - Muta. 2 - Ox. Sol. 3 - Skin Irrit. 2 - Skin Sens. 1B
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
product name: __
Quality Level: 100
product line: __
Assay: ≥95% (HPLC)
form: powder
mp: __
application(s): __
storage temp.: 2-8°C
SMILES string: CC(=O)O[C@H]1C(=O)[C@]2(C)[C@@H](O)C[C@H]3OC[C@@]3(OC(C)=O)[C@H]2[C@H](OC(=O)c4ccccc4)[C@]5(O)C[C@H](O)C(C)=C1C5(C)C
InChI: 1S/C31H38O11/c1-15-19(34)13-31(38)26(41-27(37)18-10-8-7-9-11-18)24-29(6,20(35)12-21-30(24,14-39-21)42-17(3)33)25(36)23(40-16(2)32)22(15)28(31,4)5/h7-11,19-21,23-24,26,34-35,38H,12-14H2,1-6H3/t19-,20-,21+,23+,24-,26-,29+,30-,31+/m0/s1
product name:
__
Quality Level:
100
product line:
__
Assay:
≥95% (HPLC)
form:
powder
mp:
__
application(s):
__
storage temp.:
2-8°C
SMILES string:
CC(=O)O[C@H]1C(=O)[C@]2(C)[C@@H](O)C[C@H]3OC[C@@]3(OC(C)=O)[C@H]2[C@H](OC(=O)c4ccccc4)[C@]5(O)C[C@H](O)C(C)=C1C5(C)C
InChI:
1S/C31H38O11/c1-15-19(34)13-31(38)26(41-27(37)18-10-8-7-9-11-18)24-29(6,20(35)12-21-30(24,14-39-21)42-17(3)33)25(36)23(40-16(2)32)22(15)28(31,4)5/h7-11,19-21,23-24,26,34-35,38H,12-14H2,1-6H3/t19-,20-,21+,23+,24-,26-,29+,30-,31+/m0/s1
product name: __
Quality Level: 200
product line: __
Assay: __
form: tablet
mp: __
application(s): __
storage temp.: −20°C
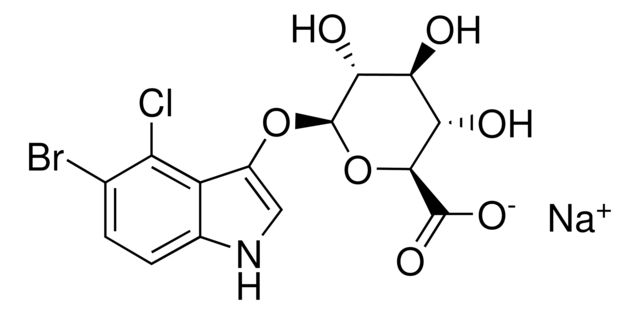
SMILES string: [Na+].O[C@@H]1[C@@H](O)[C@@H](O[C@@H]([C@H]1O)C([O-])=O)Oc2c[nH]c3ccc(Br)c(Cl)c23
InChI: 1S/C14H13BrClNO7.Na/c15-4-1-2-5-7(8(4)16)6(3-17-5)23-14-11(20)9(18)10(19)12(24-14)13(21)22;/h1-3,9-12,14,17-20H,(H,21,22);/q;+1/p-1/t9-,10-,11+,12-,14+;/m0./s1
product name:
__
Quality Level:
200
product line:
__
Assay:
__
form:
tablet
mp:
__
application(s):
__
storage temp.:
−20°C
SMILES string:
[Na+].O[C@@H]1[C@@H](O)[C@@H](O[C@@H]([C@H]1O)C([O-])=O)Oc2c[nH]c3ccc(Br)c(Cl)c23
InChI:
1S/C14H13BrClNO7.Na/c15-4-1-2-5-7(8(4)16)6(3-17-5)23-14-11(20)9(18)10(19)12(24-14)13(21)22;/h1-3,9-12,14,17-20H,(H,21,22);/q;+1/p-1/t9-,10-,11+,12-,14+;/m0./s1