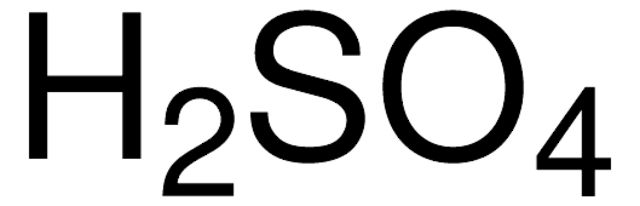143007
Hydrochloric acid
c(HCL) = 6.0 M (6.0 N)
Manufacturer: Supelco
Synonym(S): HCl Aqueous Solution
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 L | 143007-1-L | In Stock | ₹ 3,160.00 |
143007 - 1 L
In Stock
Quantity
1
Base Price: ₹ 3,160.00
GST (18%): ₹ 568.80
Total Price: ₹ 3,728.80
product name
Hydrochloric acid solution, c(HCL) = 6.0 mol/l (6.0 N)
Agency
suitable for EPA 1613
form
liquid
reaction suitability
reaction type: Acid-base reactions
concentration
6 M
technique(s)
titration: suitable
pH
<1 (20 °C in H2O)
density
1.1 g/cm3 at 20 °C
storage temp.
2-30°C
Description
- General description: c(HCL) = 6.0 mol/l (6.0 N)
- Application: Synthesis of Zinc Oxide Nanorods from Zinc Borate Precursor: This study uses hydrochloric acid in the synthesis of zinc oxide nanorods, important for supercapacitor applications. It underscores the critical role of hydrochloric acid in nanomaterials research, pushing the boundaries of energy storage technology (Lefdhil et al., 2023). An improved digestion and analysis procedure for silicon in plant tissue: Hydrochloric acid is employed to digest plant tissues for silicon content analysis, enhancing agricultural and biological research methodologies. This application reflects the importance of hydrochloric acid in scientific studies that require precise chemical processing (Langenfeld and Bugbee, 2023). N-N(+) Bond-Forming Intramolecular Cyclization of O-Tosyloxy β-Aminopropioamidoximes and Ion Exchange Reaction: In this research, hydrochloric acid plays a key role in organic synthesis, demonstrating its versatility in pharmaceutical compound development. The study provides insights into the synthesis of complex organic compounds, which are vital in medicinal chemistry (Kayukova et al., 2023).
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P234 - P261 - P264 - P271 - P302 + P352 - P305 + P351 + P338
Hazard Classifications
Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable