1A02550
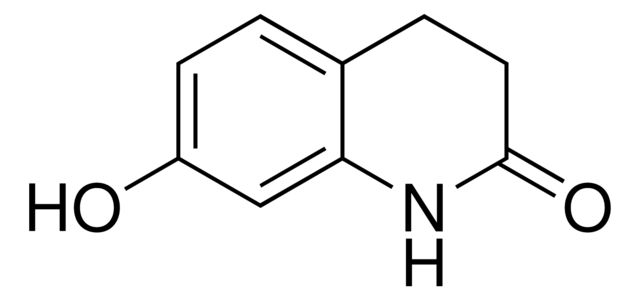
Aripiprazole Hydroxydihydroquinolinone Analog
Pharmaceutical Analytical Impurity (PAI)
Manufacturer: Sigma Aldrich
CAS Number: 22246-18-0
Synonym(S): 7-Hydroxy-3,4-dihydro-2(1H)-quinolinone, 7-Hydroxy-1,2,3,4-tetrahydro-2-quinolinone, 7-Hydroxy-3,4-dihydrocarbostyril
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 25 MG | 1A02550-25-MG | In Stock | ₹ 77,452.88 |
1A02550 - 25 MG
In Stock
Quantity
1
Base Price: ₹ 77,452.88
GST (18%): ₹ 13,941.518
Total Price: ₹ 91,394.398
grade
pharmaceutical analytical impurity (PAI)
Agency
USP
manufacturer/tradename
USP
mp
233-237 °C
application(s)
pharmaceutical
format
neat
storage temp.
2-8°C
SMILES string
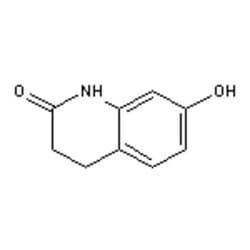
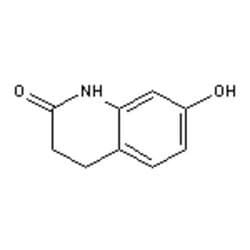
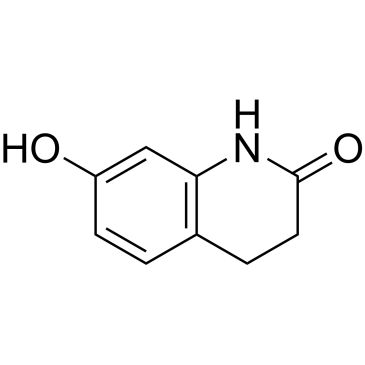
Oc1ccc2CCC(=O)Nc2c1
InChI
1S/C9H9NO2/c11-7-3-1-6-2-4-9(12)10-8(6)5-7/h1,3,5,11H,2,4H2,(H,10,12)
InChI key
LKLSFDWYIBUGNT-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Accela Chembio Inc 7-hydroxy-3 | 4-dihydro-2(1h)-quinolinone | 25g | 22246-18-0 | MFCD06410891 | 97+% | Shelf Life: 1260 Days | Light Sensitive | Accela Chembio Inc | ₹ 2,536.50 | |
 | Chem-Impex International, Inc. 7-Hydroxy-3,4-Dihydro-2(1H)-quinolinone | 22246-18-0 | MFCD06410891 | 25G | Chem-Impex International, Inc. | ₹ 4,094.89 | |
 | Sigma Aldrich Fine Chemicals Biosciences 7-Hydroxy-3,4-dihydro-2(1H)-quinolinone 97% | 22246-18-0 | MFCD06410891 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 3,987.20 | |
 | Accela Chembio Inc 7-hydroxy-3 | 4-dihydro-2(1h)-quinolinone | 100g | 22246-18-0 | MFCD06410891 | 97+% | Shelf Life: 1260 Days | Light Sensitive | Accela Chembio Inc | ₹ 4,405.50 | |
 | 3,4-Dihydro-7-Hydroxy-2(1H)-Quinolinone | Aaron Chemicals LLC | ₹ 267.00 - ₹ 12,460.00 | |
 | 7-Hydroxy-3,4-dihydro-2(1H)-quinolinone | ChemScene | ₹ 534.00 - ₹ 15,308.00 |
Description
- General description: Aripiprazole Hydroxydihydroquinolinone Analog is a USP Pharmaceutical Analytical Impurity (PAI). USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.Associated Drug Substance: Aripiprazole.For more information about this PAI, visit here.
- Application: Aripiprazole Hydroxydihydroquinolinone Analog (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
- Features and Benefits: USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:1. Conduct analytical tests during early formulation feasibility studies.2. Determine degradation impurities produced during stress studies.3. Develop, validate, and transfer analytical methods.4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.5. Record retention times and/or spectra6. Determine relative response factors.7. Identify unknown impurities that formed during ICH stability conditions.8. Identify impurities that are present in the Reference Listed Drug9. Test for and profile impurities not listed in drug substance and drug product monographs.
- Analysis Note: These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
- Other Notes: Sales restrictions may apply.
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
grade: pharmaceutical analytical impurity (PAI)
Agency: USP
manufacturer/tradename: USP
mp: 233-237 °C
application(s): pharmaceutical
format: neat
storage temp.: 2-8°C
SMILES string: Oc1ccc2CCC(=O)Nc2c1
InChI: 1S/C9H9NO2/c11-7-3-1-6-2-4-9(12)10-8(6)5-7/h1,3,5,11H,2,4H2,(H,10,12)
InChI key: LKLSFDWYIBUGNT-UHFFFAOYSA-N
grade:
pharmaceutical analytical impurity (PAI)
Agency:
USP
manufacturer/tradename:
USP
mp:
233-237 °C
application(s):
pharmaceutical
format:
neat
storage temp.:
2-8°C
SMILES string:
Oc1ccc2CCC(=O)Nc2c1
InChI:
1S/C9H9NO2/c11-7-3-1-6-2-4-9(12)10-8(6)5-7/h1,3,5,11H,2,4H2,(H,10,12)
InChI key:
LKLSFDWYIBUGNT-UHFFFAOYSA-N
grade: pharmaceutical analytical impurity (PAI)
Agency: USP
manufacturer/tradename: USP
mp: 145-148 °C (lit.)
application(s): pharmaceutical
format: neat
storage temp.: 2-8°C
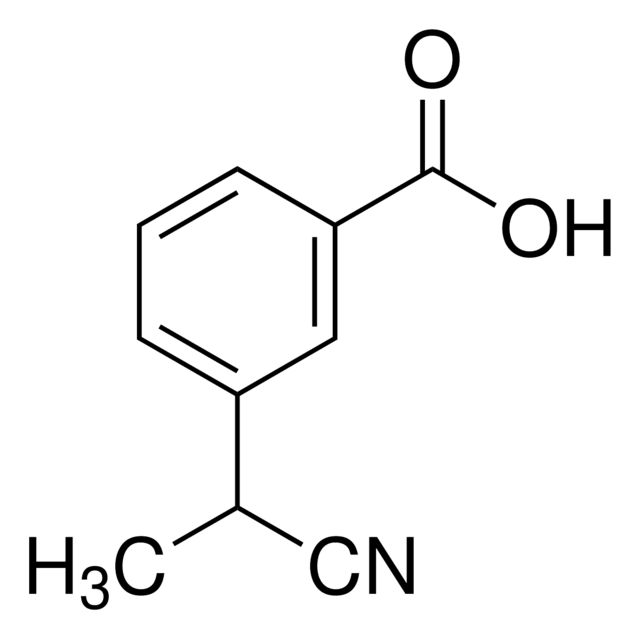
SMILES string: CC(C#N)c1cccc(c1)C(O)=O
InChI: 1S/C10H9NO2/c1-7(6-11)8-3-2-4-9(5-8)10(12)13/h2-5,7H,1H3,(H,12,13)
InChI key: IRYIYPWRXROPSX-UHFFFAOYSA-N
grade:
pharmaceutical analytical impurity (PAI)
Agency:
USP
manufacturer/tradename:
USP
mp:
145-148 °C (lit.)
application(s):
pharmaceutical
format:
neat
storage temp.:
2-8°C
SMILES string:
CC(C#N)c1cccc(c1)C(O)=O
InChI:
1S/C10H9NO2/c1-7(6-11)8-3-2-4-9(5-8)10(12)13/h2-5,7H,1H3,(H,12,13)
InChI key:
IRYIYPWRXROPSX-UHFFFAOYSA-N