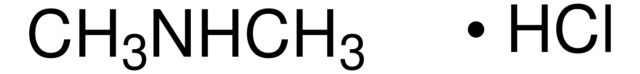PHR1525
Rabeprazole Sodium
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
CAS Number: 117976-90-6
Synonym(S): Rabeprazole sodium, 2-([4-(3-Methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl)-1H-benzo[d]imidazole, Pariprazole
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | PHR1525-1-G | In Stock | ₹ 15,880.28 |
PHR1525 - 1 G
In Stock
Quantity
1
Base Price: ₹ 15,880.28
GST (18%): ₹ 2,858.45
Total Price: ₹ 18,738.73
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
Agency
traceable to USP 1598019
API family
rabeprazole
CofA
current certificate can be downloaded
packaging
pkg of 1 g
technique(s)
HPLC: suitablegas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences Rabeprazole sodium >=98% (HPLC) | 117976-90-6 | 50MG | Sigma Aldrich Fine Chemicals Biosciences | ₹ 36,741.87 | |
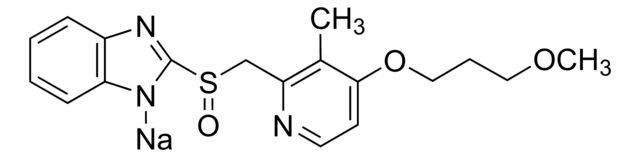 | Rabeprazole for system suitability | Sigma Aldrich | ₹ 14,072.50 | |
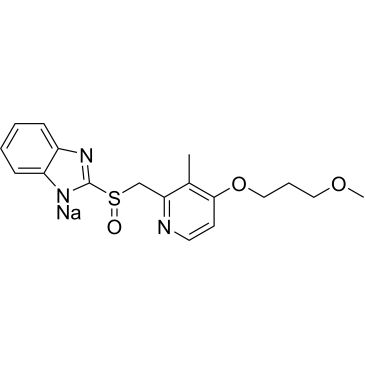 | Rabeprazole sodium | ChemScene | ₹ 6,052.00 - ₹ 65,860.00 |
Description
- General description: Rabeprazole Sodium belongs to the group of antisecretory compounds and is a proton pump inhibitor. It is widely used to suppress the gastric acid secretion by blocking the acid pump (H+ /K+ ATPase) at the secretory surface of the gastric parietal cell.[1][2]Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
- Application: Rabeprazole Sodium may be used as a pharmaceutical reference standard for the quantification of the analyte in pharmaceutical formulations[1][2] using reversed-phase high-performance liquid chromatography technique[1] and spectrophotometric technique.[3]
- Biochem/physiol Actions: Rabeprazole sodium is gastric proton pump inhibitor. It suppresses the production of acid in the stomach by inhibiting the gastric H+/K+ATPase (hydrogen-potassium adenosine triphosphatase) at the secretory surface of the gastric parietal cell. Rabeprazole sodium has been used clinically to treat acid-reflux disorders (GERD), peptic ulcer disease, H. pylori eradication, and prevent gastroinetestinal bleeds associated with NSAID use.
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Other Notes: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAA3643 in the slot below. This is an example certificate only and may not be the lot that you receive.
- Recommended products: Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 4
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to USP 1598019
API family: rabeprazole
CofA: current certificate can be downloaded
packaging: pkg of 1 g
technique(s): HPLC: suitablegas chromatography (GC): suitable
application(s): pharmaceutical (small molecule)
format: neat
storage temp.: 2-8°C
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1598019
API family:
rabeprazole
CofA:
current certificate can be downloaded
packaging:
pkg of 1 g
technique(s):
HPLC: suitablegas chromatography (GC): suitable
application(s):
pharmaceutical (small molecule)
format:
neat
storage temp.:
2-8°C
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to USP 1003031
API family: paracetamol, acetaminophen
CofA: current certificate can be downloaded
packaging: pkg of 200 mg
technique(s): HPLC: suitablegas chromatography (GC): suitable
application(s): pharmaceutical (small molecule)
format: neat
storage temp.: __
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1003031
API family:
paracetamol, acetaminophen
CofA:
current certificate can be downloaded
packaging:
pkg of 200 mg
technique(s):
HPLC: suitablegas chromatography (GC): suitable
application(s):
pharmaceutical (small molecule)
format:
neat
storage temp.:
__
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to Ph. Eur. Y0001600
API family: metformin
CofA: current certificate can be downloaded
packaging: pkg of 200 mg
technique(s): HPLC: suitablegas chromatography (GC): suitable
application(s): pharmaceutical (small molecule)
format: neat
storage temp.: __
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to Ph. Eur. Y0001600
API family:
metformin
CofA:
current certificate can be downloaded
packaging:
pkg of 200 mg
technique(s):
HPLC: suitablegas chromatography (GC): suitable
application(s):
pharmaceutical (small molecule)
format:
neat
storage temp.:
__
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to Ph. Eur. C2155000traceable to USP 1131960
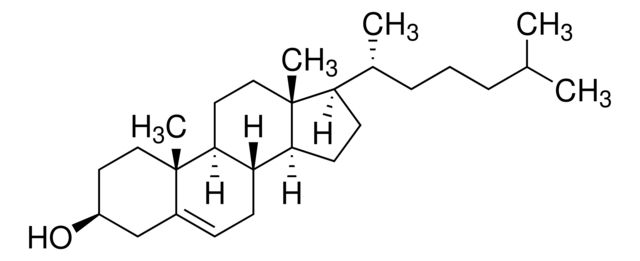
API family: cholesterol
CofA: current certificate can be downloaded
packaging: pkg of 500 mg
technique(s): HPLC: suitablegas chromatography (GC): suitable
application(s): __
format: __
storage temp.: __
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to Ph. Eur. C2155000traceable to USP 1131960
API family:
cholesterol
CofA:
current certificate can be downloaded
packaging:
pkg of 500 mg
technique(s):
HPLC: suitablegas chromatography (GC): suitable
application(s):
__
format:
__
storage temp.:
__