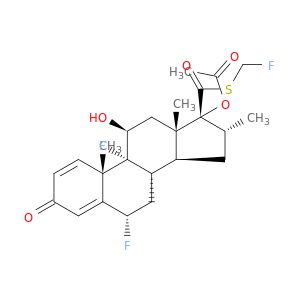
Fluticasone Related Compound C
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
CAS Number: 80474-24-4
Synonym(S): 6ALPHA,9-DIFLUORO-17- [[(FLUOROMETHYL)SULFANYL] CARBONYL]-11?ETA-HYDROXY- 16ALPHA-METHYL-3-OXOANDROSTA- 1,4-DIEN-17ALPHA-YL ACETATE (Fluticasone Related Compound C) (Fluticasone Impurity C), 6ALPHA,9-DIFLUORO-17- [[(FLUOROMETHYL)SULFANYL] CARBONYL]-11?ETA-HYDROXY- 16ALPHA-METHYL-3-OXOANDROSTA- 1,4-DIEN-17ALPHA-YL ACETATE
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 MG | PHR1843-10-MG | In Stock | ₹ 71,629.03 |
PHR1843 - 10 MG
In Stock
Quantity
1
Base Price: ₹ 71,629.03
GST (18%): ₹ 12,893.225
Total Price: ₹ 84,522.255
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
CofA
current certificate can be downloaded
packaging
pkg of 10 mg
application(s)
pharmaceutical small molecule
format
neat
storage temp.
2-8°C
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | 80474-24-4 | Fluticasone Acetate | A2B Chem | ₹ 19,580.00 - ₹ 43,610.00 |
Related Products
Description
- General description: Fluticasone Related Compound C is an impurity of the synthetic corticosteroid drug fluticasone propionate. Fluticasone propionate is generally used as an inhaled medicine for the treatment of asthma.[1][2]Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
- Application: Fluticasone Related Compound C may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by high performance liquid chromatography.[1][3]
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Other Notes: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAB4755 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable