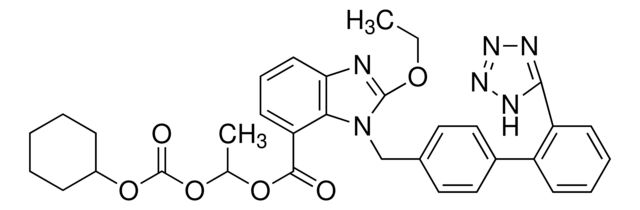
Candesartan Cilexetil
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
CAS Number: 145040-37-5
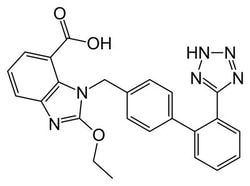
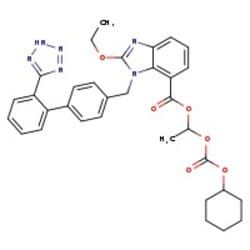
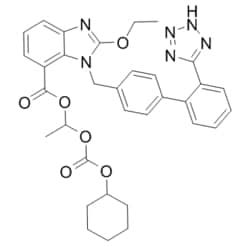
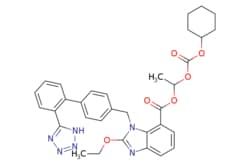
Synonym(S): Candesartan cilexetil, 2-ethoxy-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-Benzimidazole-7-carboxylic acid 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester, TCV 116, TCY 116
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 200 MG | PHR1854-200-MG | In Stock | ₹ 27,279.00 |
PHR1854 - 200 MG
In Stock
Quantity
1
Base Price: ₹ 27,279.00
GST (18%): ₹ 4,910.22
Total Price: ₹ 32,189.22
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
Agency
traceable to Ph. Eur. Y0001388traceable to USP 1087803
API family
candesartan
form
powder
packaging
pkg of 200 mg
application(s)
pharmaceutical
SMILES string
CCOc1nc2cccc(C(=O)OC(C)OC(=O)OC3CCCCC3)c2n1Cc4ccc(cc4)-c5ccccc5-c6nnn[nH]6
InChI
1S/C33H34N6O6/c1-3-42-32-34-28-15-9-14-27(31(40)43-21(2)44-33(41)45-24-10-5-4-6-11-24)29(28)39(32)20-22-16-18-23(19-17-22)25-12-7-8-13-26(25)30-35-37-38-36-30/h7-9,12-19,21,24H,3-6,10-11,20H2,1-2H3,(H,35,36,37,38)
InChI key
GHOSNRCGJFBJIB-UHFFFAOYSA-N
Other Options
Related Products
Description
- General description: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.Candesartan cilexetil is an angiotensin II receptor antagonist used as a prodrug in the treatment of hypertension.[1]
- Application: This pharmaceutical secondary standard can also be used as follows:Determination of candesartan cilexetil in tablet formulations by a UV/fluorescence spectrophotometric method[1]Study of the release of candesartan cilexetil in tablet form by reversed-phase high-performance liquid chromatography (RP-HPLC)[2]Simultaneous estimation of candesartan cilexetil and hydrochlorothiazide in pharmaceutical preparations using liquid chromatography in combination with photodiode array detector (DAD) and evaporative light scattering detector (ELSD)Spectroflourimetric determination of four angiotensin II receptor antagonists (AIIRA’s) in their pure form as well as pharmaceutical formulations
- Biochem/physiol Actions: Candesartan cilexetil is the prodrug form of the potent angiotensin II receptor antagonist, candesartan. The prodrug is cleaved by esterases within the intestine to liberate the active molecule.
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAB8504 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
- Recommended products: Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B - STOT RE 2 Oral
Target Organs
Kidney,Blood
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable