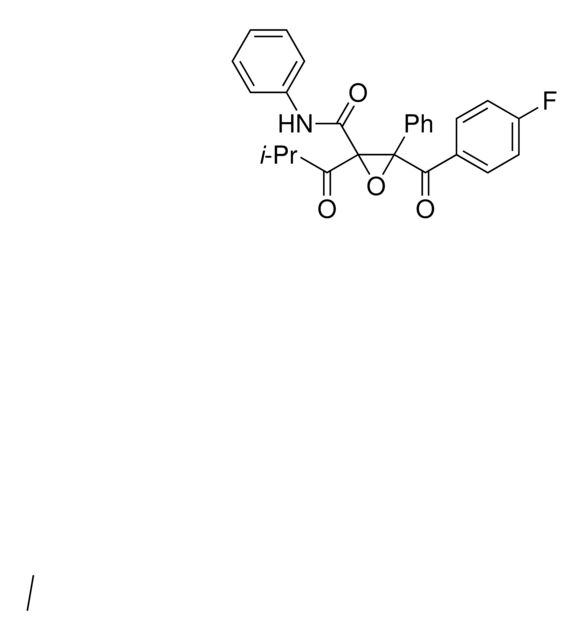
PHR1866
Ezetimibe
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
CAS Number: 163222-33-1
Synonym(S): Ezetimibe, (3R,4S)-1-(4-Fluorophenyl)-3-[(S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | PHR1866-1-G | In Stock | ₹ 27,279.00 |
PHR1866 - 1 G
In Stock
Quantity
1
Base Price: ₹ 27,279.00
GST (18%): ₹ 4,910.22
Total Price: ₹ 32,189.22
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
Agency
traceable to USP 1269028
API family
ezetimibe
form
powder
CofA
current certificate can be downloaded
packaging
pkg of 1 g
application(s)
pharmaceutical
storage temp.
2-8°C
SMILES string
FC(C=C1)=CC=C1N([C@H](C2=CC=C(O)C=C2)[C@H]3CC[C@H](O)C4=CC=C(F)C=C4)C3=O
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Apexbio Technology LLC Ezetimibe has been reported to be a cholesterol transport inhibitor. 163222-33-1. MFCD00937872 | Apexbio Technology LLC | ₹ 8,900.00 | |
 | eMolecules Ambeed / (3R4S)-1-(4-Fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)azetidin-2-one / 100mg / 518521783 / A124629 / / 163222-33-1 / MFCD00937872 / 409.433 / C24H21F2NO3 | eMolecules | ₹ 2,330.91 | |
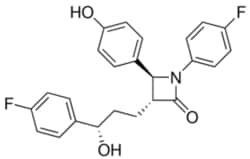 | Sigma Aldrich Fine Chemicals Biosciences Ezetimibe | 163222-33-1 | MFCD00937872 | 25mg | Sigma Aldrich Fine Chemicals Biosciences | ₹ 14,718.82 | |
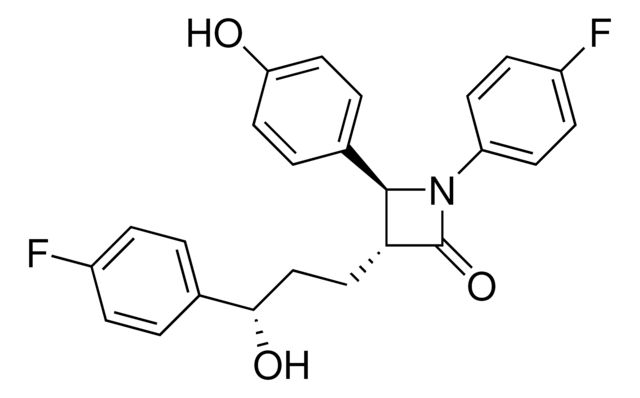 | Ezetimibe | Sigma Aldrich | ₹ 81,252.45 | |
 | 2-Azetidinone, 1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-, (3R,4S)- | Aaron Chemicals LLC | ₹ 445.00 - ₹ 30,883.00 |
Related Products
Description
- General description: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.Ezetimibe belongs to the class of 2-azetidione cholesterol absorption inhibitors. It selectively blocks the absorption of cholesterol from dietary and biliary sources and thereby prevents the transport of cholesterol through the intestinal wall.
- Application: This pharmaceutical secondary standard can also be used as follows:Development of two spectrophotometric methods to determine ezetimibe in bulk and pharmaceutical formulations[1]Separation and determination of ezetimibe and atorvastatin in their tablet dosage forms using capillary electrophoresis (CE)Simultaneous estimation of valsartan and ezetimibe in their combined dosage tablet by a stability-indicating reversed-phase high-performance liquid chromatographic (RP-HPLC) method, validated as per ICH guidelines[2]RP-HPLC method-based multi analysis of rosuvastatin, telmisartan, ezetimibe, and atorvastatin in pharmaceutical dosage formulations[3]Quantitative analysis of ezetimibe and atorvastatin calcium in pharmaceutical formulations using spectrophotometry and TLC-densitometry-based methods
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAC1517 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to USP 1269028
API family: ezetimibe
form: powder
CofA: current certificate can be downloaded
packaging: pkg of 1 g
application(s): pharmaceutical
storage temp.: 2-8°C
SMILES string: FC(C=C1)=CC=C1N([C@H](C2=CC=C(O)C=C2)[C@H]3CC[C@H](O)C4=CC=C(F)C=C4)C3=O
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1269028
API family:
ezetimibe
form:
powder
CofA:
current certificate can be downloaded
packaging:
pkg of 1 g
application(s):
pharmaceutical
storage temp.:
2-8°C
SMILES string:
FC(C=C1)=CC=C1N([C@H](C2=CC=C(O)C=C2)[C@H]3CC[C@H](O)C4=CC=C(F)C=C4)C3=O
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to Ph. Eur. Y0001330traceable to USP 1044549
API family: atorvastatin
form: __
CofA: current certificate can be downloaded
packaging: pkg of 30 mg
application(s): pharmaceutical
storage temp.: -10 to -25°C
SMILES string: __
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to Ph. Eur. Y0001330traceable to USP 1044549
API family:
atorvastatin
form:
__
CofA:
current certificate can be downloaded
packaging:
pkg of 30 mg
application(s):
pharmaceutical
storage temp.:
-10 to -25°C
SMILES string:
__
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to Ph. Eur. Y0001331traceable to USP 1044550
API family: atorvastatin
form: __
CofA: current certificate can be downloaded
packaging: pkg of 30 mg
application(s): pharmaceutical
storage temp.: 2-30°C
SMILES string: __
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to Ph. Eur. Y0001331traceable to USP 1044550
API family:
atorvastatin
form:
__
CofA:
current certificate can be downloaded
packaging:
pkg of 30 mg
application(s):
pharmaceutical
storage temp.:
2-30°C
SMILES string:
__