PHR1904
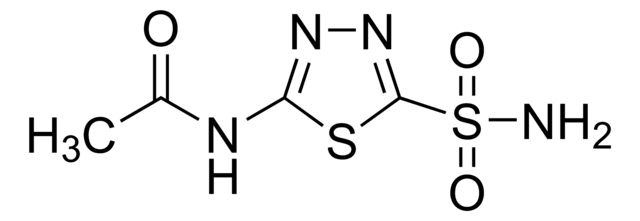
Citalopram Related Compound E
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
Synonym(S): 1-(3-Dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile N-oxide hydrochloride, 1-(3-DIMETHYLAMINOPROPYL)-1-(4-FLUOROPHENYL)-1,3-DIHYDROISOBENZOFURAN-5-CARBONITRILE-N-OXIDE HYDROCHLORIDE
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 50 MG | PHR1904-50-MG | In Stock | ₹ 75,937.38 |
PHR1904 - 50 MG
In Stock
Quantity
1
Base Price: ₹ 75,937.38
GST (18%): ₹ 13,668.728
Total Price: ₹ 89,606.108
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
Agency
traceable to USP 1134288
API family
citalopram
CofA
current certificate can be downloaded
packaging
pkg of 50 mg
application(s)
pharmaceutical small molecule
format
neat
storage temp.
2-30°C
Related Products
Description
- General description: Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Citalopram Related Compound E is a related impurity found in the drug Citalopram. Citalopram is a racemic bicyclic phthalane derivative and is a potent anti-depressant drug, that acts by inhibition of serotonin uptake.[1]
- Application: Citalopram Related Compound E may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by chromatography.[2]
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Other Notes: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAB1229 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to Ph. Eur. A0040000traceable to USP 1000601
API family: acebutolol
CofA: current certificate can be downloaded
packaging: pkg of 250 mg
application(s): pharmaceutical
format: __
storage temp.: 2-30°C
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to Ph. Eur. A0040000traceable to USP 1000601
API family:
acebutolol
CofA:
current certificate can be downloaded
packaging:
pkg of 250 mg
application(s):
pharmaceutical
format:
__
storage temp.:
2-30°C
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to BP 904traceable to Ph. Eur. A0100000traceable to USP 1005004
API family: acetazolamide
CofA: current certificate can be downloaded
packaging: pkg of 5 g
application(s): pharmaceutical
format: neat
storage temp.: 2-30°C
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to BP 904traceable to Ph. Eur. A0100000traceable to USP 1005004
API family:
acetazolamide
CofA:
current certificate can be downloaded
packaging:
pkg of 5 g
application(s):
pharmaceutical
format:
neat
storage temp.:
2-30°C