Salbutamol Impurity B
Pharmaceutical Secondary Standard; Certified Reference Material
Manufacturer: Supelco
CAS Number: 96948-64-0
Synonym(S): (1RS)-2-[(1,1-Dimethylethyl)amino]-1-(4-hydroxyphenyl)ethanol, Albuterol Related Compound I, 2-tert-Butylamino-1-(4-hydroxyphenyl)ethanol
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 50 MG | PHR1959-50-MG | In Stock | ₹ 69,074.33 |
PHR1959 - 50 MG
In Stock
Quantity
1
Base Price: ₹ 69,074.33
GST (18%): ₹ 12,433.379
Total Price: ₹ 81,507.709
grade
certified reference materialpharmaceutical secondary standard
Quality Level
300
Agency
traceable to BP 852traceable to Ph. Eur. Y0000030
form
powder
CofA
current certificate can be downloaded
packaging
pkg of 50 mg
application(s)
pharmaceutical
storage temp.
2-8°C
InChI
1S/C12H19NO2/c1-12(2,3)13-8-11(15)9-4-6-10(14)7-5-9/h4-7,11,13-15H,8H2,1-3H3
InChI key
JOGFUYPGDLRKHD-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences Salbutamol impurity B European Pharmacopoeia (EP) Reference Standard | 96948-64-0 | | Sigma Aldrich Fine Chemicals Biosciences | ₹ 24,531.07 | |
 | Salbutamol Impurity B Pharmaceutical Secondary Standard, MilliporeSigma™ Supelco™ | MilliporeSigma Supelco | ₹ 75,561.00 | |
 | Salbutamol impurity B | Sigma Aldrich | ₹ 21,401.03 | |
 | Salbutamol impurity B | Sigma Aldrich | ₹ 16,107.60 | |
 | Albuterol Related Compound I | Sigma Aldrich | ₹ 1,33,396.48 | |
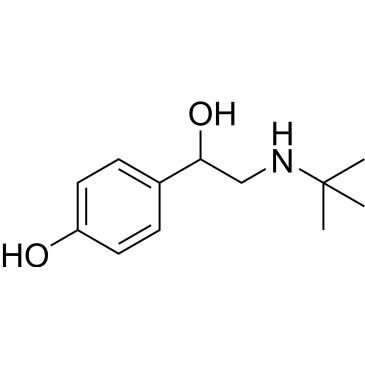 | 4-(2-(tert-Butylamino)-1-hydroxyethyl)phenol Salbutamol Impurity | ChemScene | -- | |
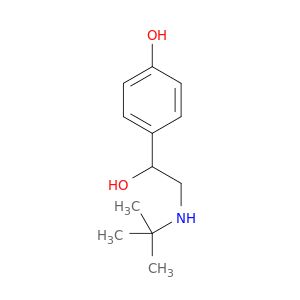 | 96948-64-0 | 4-(2-(tert-Butylamino)-1-hydroxyethyl)phenol | A2B Chem | ₹ 99,057.00 |
Related Products
Description
- General description: Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
- Application: Salbutamol Impurity B Analysis in Pharmaceutical Products: Utilizing advanced chromatographic techniques, researchers have developed validated methods for the precise identification and quantification of Salbutamol Impurity B in pharmaceutical formulations. This critical quality control measure ensures the safety and efficacy of bronchodilator medications, adhering to stringent regulatory standards (Kondra et al., 2023).Development of Analytical Standards for Salbutamol Impurity B: The quantification of Salbutamol Impurity B using achiral supercritical fluid chromatography represents a significant advancement in the field of pharmaceutical impurities identification. This method provides a robust, efficient, and reproducible approach for monitoring the stability and purity of salbutamol-based therapies, contributing to enhanced patient safety (Dispas et al., 2017).Research on Salbutamol Degradation Products: A stability-indicating RP-HPLC method has been developed for the simultaneous determination of Salbutamol Impurity B and other related substances in nasal solutions. This method is crucial for understanding the degradation pathways of salbutamol, thereby assisting in the development of more stable and effective bronchodilator formulations (Kasawar & Farooqui, 2010).
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
- Other Notes: This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
- Footnote: To see an example of a Certificate of Analysis for this material enter LRAB3289 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
- Recommended products: Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P261 - P264 - P271 - P280 - P302 + P352 - P305 + P351 + P338
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable

