PHR2912
Famotidine Related Compound E
reference material, pharmaceutical secondary standard
Manufacturer: Supelco
CAS Number: 129083-44-9
Synonym(S): N,N′′′-[Dithiobis(methylene-4,2-thiazolediyl)]bisguanidine, 2,2′-[4,4′-Disulfanediylbis(methylene)bis(thiazole-4,2-diyl)]diguanidine
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 50 MG | PHR2912-50-MG | In Stock | ₹ 74,043.00 |
PHR2912 - 50 MG
In Stock
Quantity
1
Base Price: ₹ 74,043.00
GST (18%): ₹ 13,327.74
Total Price: ₹ 87,370.74
grade
pharmaceutical secondary standardreference material
Quality Level
200
Agency
traceable to USP 1269254
packaging
pkg of 50 mg
application(s)
pharmaceutical small molecule
InChI
1S/C10H14N8S4/c11-7(12)17-9-15-5(1-19-9)3-21-22-4-6-2-20-10(16-6)18-8(13)14/h1-2H,3-4H2,(H4,11,12,15,17)(H4,13,14,16,18)
InChI key
ZWHJVLVEEDAPHN-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences Famotidine Related Compound E United States Pharmacopeia (USP) Reference Standard | 129083-44-9 | 25MG | Sigma Aldrich Fine Chemicals Biosciences | ₹ 1,57,601.20 | |
 | Famotidine Related Compound E | Sigma Aldrich | ₹ 1,39,610.03 | |
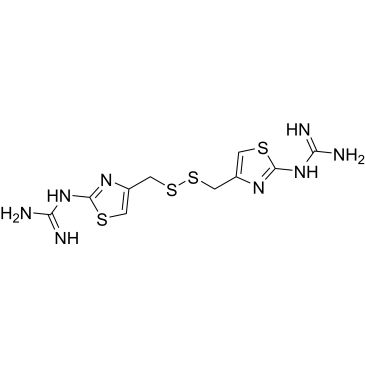 | 1,1'-(4,4'-(Disulfanediylbis(methylene))bis(thiazole-4,2-diyl))diguanidine Famotidine Impurity | ChemScene | -- | |
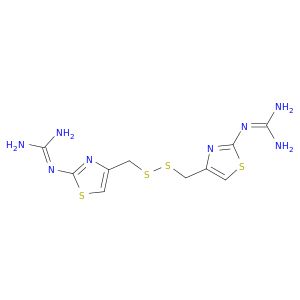 | 129083-44-9 | Bis[(2-guanidino-4-thiazolyl)methyl]disulfide | A2B Chem | ₹ 99,057.00 |
Related Products
Description
- General description: Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
- Application: These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
- Analysis Note: These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
grade: pharmaceutical secondary standardreference material
Quality Level: 200
Agency: traceable to USP 1269254
packaging: pkg of 50 mg
application(s): pharmaceutical small molecule
InChI: 1S/C10H14N8S4/c11-7(12)17-9-15-5(1-19-9)3-21-22-4-6-2-20-10(16-6)18-8(13)14/h1-2H,3-4H2,(H4,11,12,15,17)(H4,13,14,16,18)
InChI key: ZWHJVLVEEDAPHN-UHFFFAOYSA-N
grade:
pharmaceutical secondary standardreference material
Quality Level:
200
Agency:
traceable to USP 1269254
packaging:
pkg of 50 mg
application(s):
pharmaceutical small molecule
InChI:
1S/C10H14N8S4/c11-7(12)17-9-15-5(1-19-9)3-21-22-4-6-2-20-10(16-6)18-8(13)14/h1-2H,3-4H2,(H4,11,12,15,17)(H4,13,14,16,18)
InChI key:
ZWHJVLVEEDAPHN-UHFFFAOYSA-N
grade: certified reference materialpharmaceutical secondary standard
Quality Level: 300
Agency: traceable to USP 1119309
packaging: pkg of 50 mg
application(s): pharmaceutical
InChI: 1S/C14H10ClNO5S/c15-11-6-5-8(7-12(11)22(16,20)21)13(17)9-3-1-2-4-10(9)14(18)19/h1-7H,(H,18,19)(H2,16,20,21)
InChI key: UKRADWWBRBBJLZ-UHFFFAOYSA-N
grade:
certified reference materialpharmaceutical secondary standard
Quality Level:
300
Agency:
traceable to USP 1119309
packaging:
pkg of 50 mg
application(s):
pharmaceutical
InChI:
1S/C14H10ClNO5S/c15-11-6-5-8(7-12(11)22(16,20)21)13(17)9-3-1-2-4-10(9)14(18)19/h1-7H,(H,18,19)(H2,16,20,21)
InChI key:
UKRADWWBRBBJLZ-UHFFFAOYSA-N

