D4407
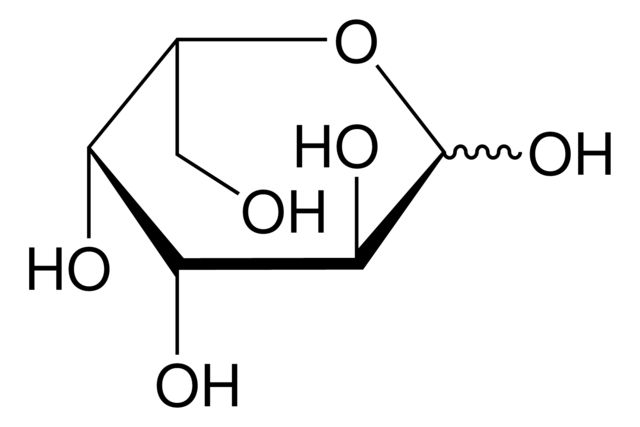
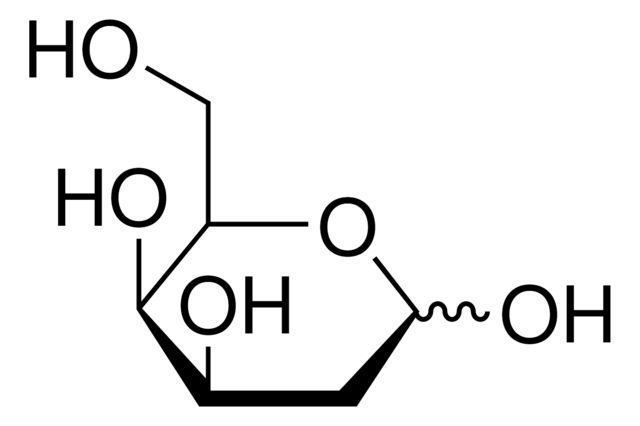
2-Deoxy-D-galactose
98%
Manufacturer: Sigma Aldrich
CAS Number: 1949-89-9
Synonym(S): 2-Deoxy-D-lyxohexose
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | D4407-1-G | In Stock | ₹ 7,317.70 |
| 5 G | D4407-5-G | In Stock | ₹ 23,046.43 |
D4407 - 1 G
In Stock
Quantity
1
Base Price: ₹ 7,317.70
GST (18%): ₹ 1,317.186
Total Price: ₹ 8,634.886
Quality Level
200
Assay
98%
form
powder
optical activity
[α]20/D +59.7°, c = 2 in H2O
color
white to off-white
mp
107 - 110 °C ((225 - 230 °F))107-110 °C (lit.)
SMILES string
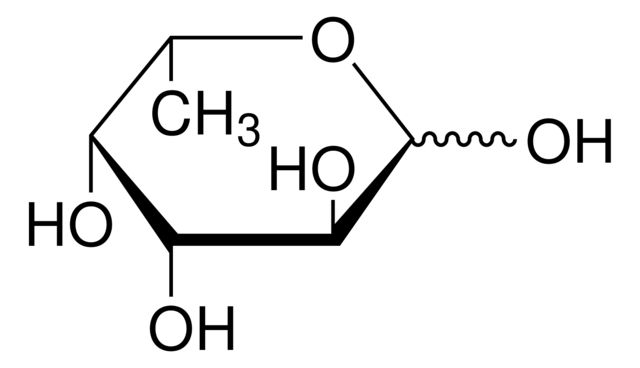
OC[C@H]1OC(O)C[C@@H](O)[C@H]1O
InChI
1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2/t3-,4-,5?,6-/m1/s1
InChI key
PMMURAAUARKVCB-DUVQVXGLSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences 2-Deoxy-D-galactose 98% | 1949-89-9 | MFCD00014649 | 5G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 31,726.72 | |
 | 2-Deoxy-D-galactose | Sigma Aldrich | ₹ 7,317.70 - ₹ 23,046.43 | |
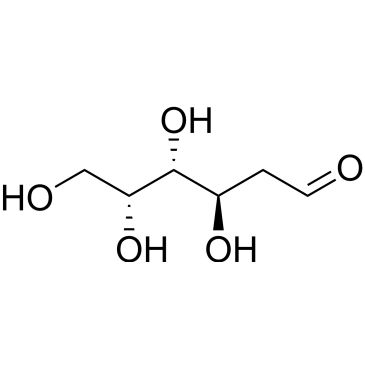 | 2-Deoxy-D-galactose | ChemScene | ₹ 8,010.00 - ₹ 74,315.00 | |
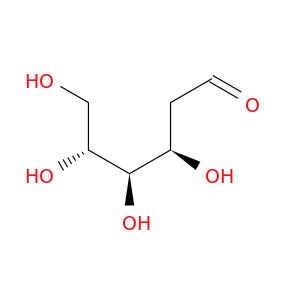 | 1949-89-9 | 2-Deoxy-d-galactose | A2B Chem | ₹ 6,942.00 - ₹ 81,168.00 |
Related Products
Description
- General description: 2-Deoxy-D-galactose is a glucose analog that shows a wide range of biological activities such as inhibition of glycolysis and thereby tumor growth, interference with the biosynthetic processing of glycoproteins, antiviral activity, and hepatotoxicity. It is being extensively studied as trapping agents for phosphate and uridylate in mammalian cells due to its ability to interfere in the phosphate and nucleotide metabolism.[1][2][3]
- Application: FUT1-mediated terminal fucosylation acts as a new target to attenuate renal fibrosis.: This research investigates the role of 2-deoxy-D-galactose in modulating terminal fucosylation processes, revealing potential therapeutic pathways for treating renal fibrosis and enhancing the understanding of kidney disease mechanisms (Luo et al., 2023).2-D-gal Targets Terminal Fucosylation to Inhibit T-cell Response in a Mouse Skin Transplant Model.: Highlights the immunomodulatory potential of 2-deoxy-D-galactose in transplant medicine, showing how it can inhibit T-cell responses and contribute to the success of skin grafts, pointing towards new immunosuppressive treatments (Mao et al., 2023). Inhibition of Aberrant α(1,2)-Fucosylation at Ocular Surface Ameliorates Dry Eye Disease.: Explores the therapeutic effects of 2-deoxy-D-galactose in treating dry eye disease by modulating specific fucosylation pathways, potentially opening new avenues for ocular surface treatment strategies (Yoon et al., 2021).
- Other Notes: To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
Quality Level: 200
Assay: 98%
form: powder
optical activity: [α]20/D +59.7°, c = 2 in H2O
color: white to off-white
mp: 107 - 110 °C ((225 - 230 °F))107-110 °C (lit.)
SMILES string: OC[C@H]1OC(O)C[C@@H](O)[C@H]1O
InChI: 1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2/t3-,4-,5?,6-/m1/s1
InChI key: PMMURAAUARKVCB-DUVQVXGLSA-N
Quality Level:
200
Assay:
98%
form:
powder
optical activity:
[α]20/D +59.7°, c = 2 in H2O
color:
white to off-white
mp:
107 - 110 °C ((225 - 230 °F))107-110 °C (lit.)
SMILES string:
OC[C@H]1OC(O)C[C@@H](O)[C@H]1O
InChI:
1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2/t3-,4-,5?,6-/m1/s1
InChI key:
PMMURAAUARKVCB-DUVQVXGLSA-N