108958
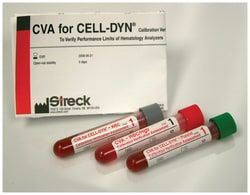
Streck CVA™ Calibration Verification Assessment/Linearity Kit
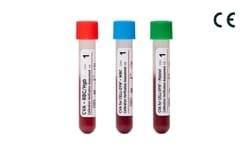
CVA (Calibration Verification Assessment) and CVA for CELL-DYN are assayed linearity control kits that test the upper and lower limits of three- and five-part differential hematology analyzers to determine their patient reportable range and linear performance.
Manufacturer: Fischer Scientific
The price for this product is unavailable. Please request a quote
Type
Linearity Control Kit
Includes
WBC, platelet and RBC or Hemoglobin vials
Level
5
Stability
5 Days Open, 120 Days Closed Vial
Description
- Provides assay values for the most popular three- and five-part hematology instruments
- Customized kits with WBC, RBC, Hgb and platelet ranges for each instrument model
- Plastic vials with pierceable caps for analyzer autosampling
- 5-day open-vial stability, 120-day closed-vial stability
- Tests the extreme upper and lower limits of an instrument
- Access to STATS™, Streck’s free interlaboratory quality control program for peer group data comparison.