7191956
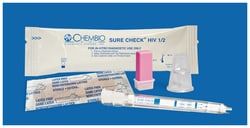
OraSure Technologies OraQuick™ Advance™ Rapid HIV-1/2 Antibody Test
HIV Testing anytime, anywhere, made easy. The OraQuick™ ADVANCE™ Rapid HIV-1/2 Antibody Test is an easy-to-use rapid HIV test that offers several specimen types - oral fluid, fingerstick whole blood, venipuncture whole blood and plasma. Yielding accurate results in as little as 20 minutes.
Manufacturer: Fischer Scientific
The price for this product is unavailable. Please request a quote
Certifications/Compliance
ISO 13485 Certified. FDA approved under PMA BP010047. Inspected to 21 CFR 820 and MDSAP certification.
Description
Detects HIV-1 and HIV-2. It is developed with synthetic peptides that represent the envelope region.
Detectable Analytes
HIV-1/2
Content And Storage
2°C to 27°C
CE Marker
Yes
Disposable
Single-use
Description
- The OraQuick™ ADVANCE™ Rapid HIV-1/2 Antibody Test detects antibodies to HIV-1 and HIV-2 in 20 minutes
- Approved for oral fluid, plasma, fingerstick or venipuncture whole blood specimens
- CLIA-waived for oral fluid, fingerstick and venipuncture whole blood and offers the ability to test in non-traditional testing environments, such as outreach programs and mobile testing clinics
- CLIA Moderately Complex for plasma samples
- The OraQuick™ ADVANCE™ is comprised of a single-use test device and a single-use vial containing a pre-measured amount of a buffered developer solution
- Each component is sealed in separate compartments of a single pouch to form the test
- 25 Test count packaging includes: (25) Divided Pouches with Test Device and 1 mL Vial Developer Solution, (5) Reusable Test Stands, (25) Specimen Collection Loops, (25) Information Pamphlets, Package Insert, Customer Letter
- 100 Test count packaging includes: (100) Divided pouches with (1) test device, (1) absorbent pack, (1) 1 mL developer solution vial; (10) reusable test stands, (100) specimen collection loops, (100) subject information pamphlets, (1) package insert, (1) customer letter
- Store unused tests at 2 to 27°C (36-80°F)
- If stored refrigerated, ensure that the divided pouch is brought to operating temperature (15-37°C, 59-99°F) before opening.
Compare Similar Items
Show Difference
Certifications/Compliance: ISO 13485 Certified. FDA approved under PMA BP010047. Inspected to 21 CFR 820 and MDSAP certification.
Description: Detects HIV-1 and HIV-2. It is developed with synthetic peptides that represent the envelope region.
Detectable Analytes: HIV-1/2
Content And Storage: 2°C to 27°C
CE Marker: Yes
Disposable: Single-use
Certifications/Compliance:
ISO 13485 Certified. FDA approved under PMA BP010047. Inspected to 21 CFR 820 and MDSAP certification.
Description:
Detects HIV-1 and HIV-2. It is developed with synthetic peptides that represent the envelope region.
Detectable Analytes:
HIV-1/2
Content And Storage:
2°C to 27°C
CE Marker:
Yes
Disposable:
Single-use