A3830802
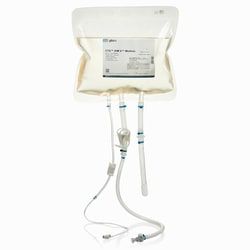
Gibco™ CTS™ AIM-V™ Medium, without phenol red, without antibiotics
Manufacturer: Fischer Scientific
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| Each of 1 | A3830802-Each-of-1 | In Stock | ₹ 1,38,128.00 |
A3830802 - Each of 1
In Stock
Quantity
1
Base Price: ₹ 1,38,128.00
GST (18%): ₹ 24,863.04
Total Price: ₹ 1,62,991.04
Content And Storage
Store at 2–8°C and protect from light.
Classification
Animal Origin
Product Type
Cell Culture Medium
Sterility
Sterile-filtered
Manufacturing Quality
ISO 13485, MDSAP, FDA-registered, 21 CFR 820
Shipping Condition
Ambient
Cell Type
Monocytes, Dendritic Cells, T Cells, Hybridomas, PBMCs, Fibroblasts, Macrophages, Myeloma Cells, Lymphocytes
Form
Liquid
Serum Level
Serum-free
Format
BPC
Quantity
10 L
Description
- Gibco Cell Therapy Systems (CTS) AIM-V Medium is the first commercially available, defined, serum-free medium (SFM) for the proliferation and/or manipulation of T cells and dendritic cells manufactured in compliance with cGMP
- It contains L-glutamine, but phenol red, gentamicin sulfate, and streptomycin sulfate have been removed to meet global regulatory requirements for ancillary materials used in cell therapy manufacturing
- CTS AIM-V Medium can be supplemented with human AB serum or CTS Immune Cell SR and various cytokines or growth factors depending on the application
- cGMP manufactured in compliance with 21 CFR part 820 Supports expansion of human peripheral blood T-cells including CAR T cells Supports expansion of activated human dendritic cells and other immune cells Proven clinical use as an ancillary reagent in Provenge™ (sipuleucel-T), the first therapeutic cancer vaccine approved by the FDA (1) Adherence to supplier-related responsibilities of USP <1043>* Closed systems compatible For convenience, CTS AIM-V Medium is available in three formats: a 1000-mL bottle for small volume users, a 10-L bag for larger volume users, and a 2-L bioprocess container designed for users interested in a closed system workflow
- The BPC chamber is constructed from Aegis 5-14 film and includes sterile, weldable DEPH-free PVC tubing with Luer lock and C-Flex™ tubing with MPC quick connector providing versatile connection options for your manufacturing process
- Security of supply CTS AIM-V Medium is a defined serum-free formulation and can be manufactured at scale required to meet future clinical and commercial demand
- Alternate manufacturing sites are available as further risk mitigation
- CTS quality Gibco CTS products are manufactured at a site that uses methods and controls that conform to cGMP for medical devices, 21 CFR Part 820
- Our FDA-registered manufacturing sites are ISO 13485-certified
- Gibco CTS products follow USP <1043> 'ancillary materials for cell, gene, and tissue-engineered products' within the responsibilities applicable to a supplier.* Accompanied by documentation to support your regulatory filing Specific intended-use statements, full documentation traceability, and convenient access to our Drug Master File (DMF) are available
- Immune cell applications CTS AIM-V Medium is cited in many peer-reviewed journals and used in multiple immune cell clinical applications such as activated dendritic cells
- It supports expansion of T cells, T cell subsets, and virus-specific T cells
- The medium can also be used to support natural killer cells combined with feeders
- Each lot of CTS AIM-V SFM is pre-qualified using Jurkat cells, an immortalized human T lymphocyte line, to help ensure consistent performance
- *Other aspects of USP <1043> will be the responsibility of the end-user to assess
- Thermo Fisher Scientific cannot fulfill USP <1043> in regards to application and therapy-specific aspects (e.g., residual assessment and removal of the AM)
- 1
- Madan RA, Gulley JL
- Sipuleucel-T: harbinger of a new age of therapeutics for prostate cancer
- Expert Rev Vaccines
- 2011 10(2):141-50.
SAFETY INFORMATION
- ShelfLife : 14 months from date of manufacture
Compare Similar Items
Show Difference
Content And Storage: Store at 2–8°C and protect from light.
Classification: Animal Origin
Product Type: Cell Culture Medium
Sterility: Sterile-filtered
Manufacturing Quality: ISO 13485, MDSAP, FDA-registered, 21 CFR 820
Shipping Condition: Ambient
Cell Type: Monocytes, Dendritic Cells, T Cells, Hybridomas, PBMCs, Fibroblasts, Macrophages, Myeloma Cells, Lymphocytes
Form: Liquid
Serum Level: Serum-free
Format: BPC
Quantity: 10 L
Content And Storage:
Store at 2–8°C and protect from light.
Classification:
Animal Origin
Product Type:
Cell Culture Medium
Sterility:
Sterile-filtered
Manufacturing Quality:
ISO 13485, MDSAP, FDA-registered, 21 CFR 820
Shipping Condition:
Ambient
Cell Type:
Monocytes, Dendritic Cells, T Cells, Hybridomas, PBMCs, Fibroblasts, Macrophages, Myeloma Cells, Lymphocytes
Form:
Liquid
Serum Level:
Serum-free
Format:
BPC
Quantity:
10 L