47627
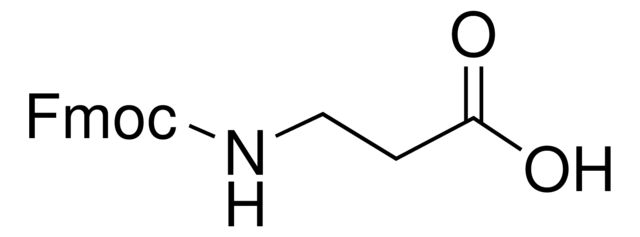
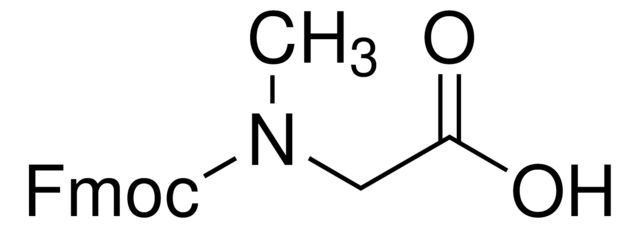
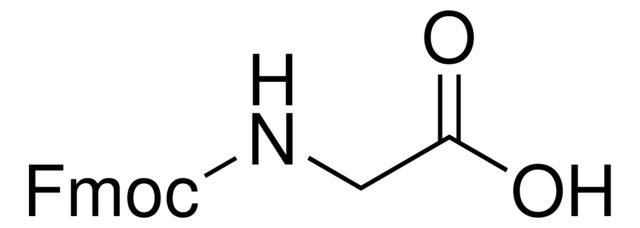
Fmoc-Gly-OH
≥98.0% (T)
Manufacturer: Sigma Aldrich
CAS Number: 29022-11-5
Synonym(S): Fmoc-glycine
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 G | 47627-5-G | In Stock | ₹ 2,533.05 |
| 50 G | 47627-50-G | In Stock | ₹ 8,324.43 |
| 250 G | 47627-250-G | In Stock | ₹ 16,843.70 |
| 1 KG | 47627-1-KG | In Stock | ₹ 33,817.30 |
47627 - 5 G
In Stock
Quantity
1
Base Price: ₹ 2,533.05
GST (18%): ₹ 455.949
Total Price: ₹ 2,988.999
Quality Level
100
Assay
≥98.0% (T)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
mp
174-175 °C (lit.)174-178 °C
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
OC(=O)CNC(=O)OCC1c2ccccc2-c3ccccc13
InChI
1S/C17H15NO4/c19-16(20)9-18-17(21)22-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H,18,21)(H,19,20)
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Chem-Impex International, Inc. Fmoc-glycine | 29022-11-5 | MFCD00037140 | 25G | Chem-Impex International, Inc. | ₹ 3,493.25 | |
 | Chem-Impex International, Inc. Fmoc-glycine | 29022-11-5 | MFCD00037140 | 5G | Chem-Impex International, Inc. | ₹ 3,003.75 | |
 | Protein Technologies Inc Fmoc-Gly-OH | 29022-11-5 | 297.30 g/mol | 1KG | Protein Technologies Inc | ₹ 53,934.00 | |
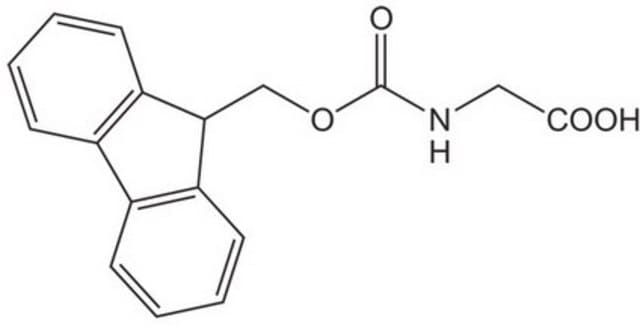 | Fmoc-Gly-OH | Sigma Aldrich | ₹ 3,360.00 - ₹ 18,940.00 | |
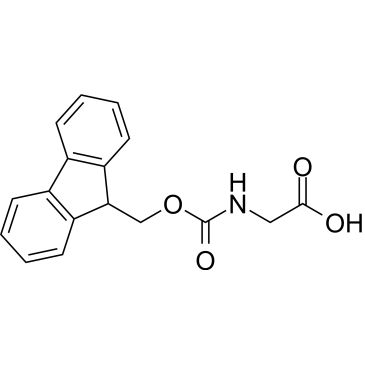 | Fmoc-Gly-OH | ChemScene | ₹ 445.00 - ₹ 20,025.00 | |
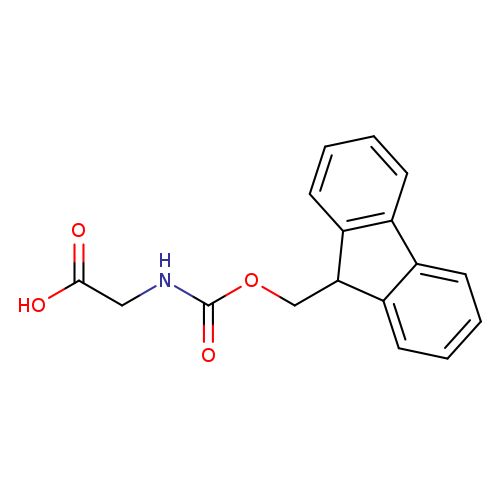 | 29022-11-5 | Fmoc-Gly-OH | A2B Chem | ₹ 356.00 - ₹ 8,633.00 |
Description
- Application: Efficient Fmoc-Protected Amino Ester Hydrolysis Using Green Calcium (II) Iodide as a Protective Agent: This study describes the utilization of environmentally friendly calcium iodide in the hydrolysis of Fmoc-protected amino esters, enhancing reaction efficiency and sustainability (R Binette, M Desgagné, C Theaud, PL Boudreault - Molecules, 2022). Link to the article.α/β-Chimera peptide synthesis with cyclic β-sugar amino acids: the efficient coupling protocol: This research provides an advanced synthesis method for α/β-chimera peptides using cyclic β-sugar amino acids, demonstrating significant implications for peptide design in medicinal chemistry (A Nagy, V Goldschmidt Gőz, I Pintér, V Farkas - Amino acids, 2019). Link to the article.MS, CD, and FT-IR characterization of five newly synthesized histidine-containing Ala-and Gly-based peptides: This paper presents detailed characterization of novel histidine-containing peptides, highlighting techniques that could be pivotal for peptide-based drug discovery (M Murariu, L Ion, CI Ciobanu, BA Petre - Rev. Roum Chem., 2017). Link to the article.Efficient method for the concentration determination of fmoc groups incorporated in the core-shell materials by Fmoc–glycine: This article elaborates on an efficient method for determining the concentration of fmoc groups in core-shell materials, critical for the design of advanced functional materials (E Szczepańska, B Grobelna, J Ryl, A Kulpa - Molecules, 2020). Link to the article.Circular aqueous fmoc/t‐bu solid‐phase peptide synthesis: This study explores a novel approach in solid-phase peptide synthesis, utilizing circular aqueous techniques that may offer greener and more efficient methodologies for peptide synthesis (J Pawlas, JH Rasmussen - ChemSusChem, 2021). Link to the article.
- Other Notes: Fmoc-glycine coupling of saccharide β-glycosylamines for the fractionation of oligosaccharides and formation of neoglycoconjugates.[1]
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
Quality Level: 100
Assay: ≥98.0% (T)
form: powder
reaction suitability: reaction type: Fmoc solid-phase peptide synthesis
mp: 174-175 °C (lit.)174-178 °C
application(s): peptide synthesis
functional group: Fmoc
storage temp.: 2-8°C
SMILES string: OC(=O)CNC(=O)OCC1c2ccccc2-c3ccccc13
InChI: 1S/C17H15NO4/c19-16(20)9-18-17(21)22-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H,18,21)(H,19,20)
Quality Level:
100
Assay:
≥98.0% (T)
form:
powder
reaction suitability:
reaction type: Fmoc solid-phase peptide synthesis
mp:
174-175 °C (lit.)174-178 °C
application(s):
peptide synthesis
functional group:
Fmoc
storage temp.:
2-8°C
SMILES string:
OC(=O)CNC(=O)OCC1c2ccccc2-c3ccccc13
InChI:
1S/C17H15NO4/c19-16(20)9-18-17(21)22-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H,18,21)(H,19,20)
Quality Level: 100
Assay: ≥98.0% (T)
form: solid
reaction suitability: reaction type: C-H Activationreaction type: Fmoc solid-phase peptide synthesisreagent type: ligandreaction type: Peptide Synthesis
mp: 145-147 °C (lit.)
application(s): peptide synthesis
functional group: Fmocaminecarboxylic acid
storage temp.: 2-8°C
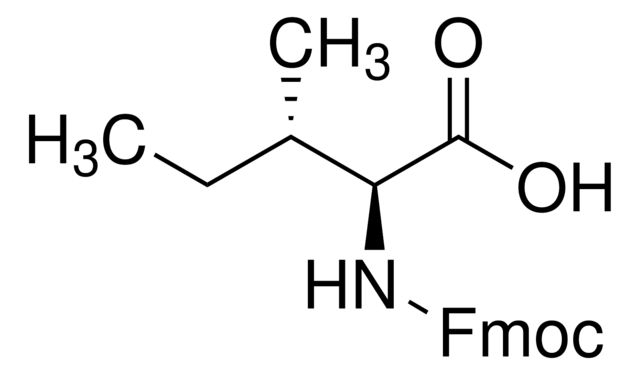
SMILES string: CC[C@H](C)[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI: __
Quality Level:
100
Assay:
≥98.0% (T)
form:
solid
reaction suitability:
reaction type: C-H Activationreaction type: Fmoc solid-phase peptide synthesisreagent type: ligandreaction type: Peptide Synthesis
mp:
145-147 °C (lit.)
application(s):
peptide synthesis
functional group:
Fmocaminecarboxylic acid
storage temp.:
2-8°C
SMILES string:
CC[C@H](C)[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI:
__
Quality Level: 100
Assay: ≥95.0% (HPLC)
form: __
reaction suitability: reaction type: Fmoc solid-phase peptide synthesis
mp: 73-77 °C
application(s): peptide synthesis
functional group: Fmoc
storage temp.: 2-8°C
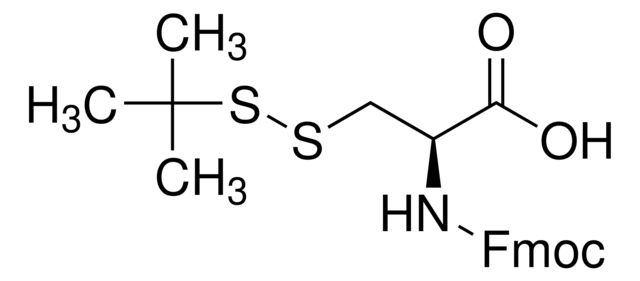
SMILES string: CC(C)(C)SSC[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI: 1S/C22H25NO4S2/c1-22(2,3)29-28-13-19(20(24)25)23-21(26)27-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,23,26)(H,24,25)/t19-/m0/s1
Quality Level:
100
Assay:
≥95.0% (HPLC)
form:
__
reaction suitability:
reaction type: Fmoc solid-phase peptide synthesis
mp:
73-77 °C
application(s):
peptide synthesis
functional group:
Fmoc
storage temp.:
2-8°C
SMILES string:
CC(C)(C)SSC[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI:
1S/C22H25NO4S2/c1-22(2,3)29-28-13-19(20(24)25)23-21(26)27-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,23,26)(H,24,25)/t19-/m0/s1