121967
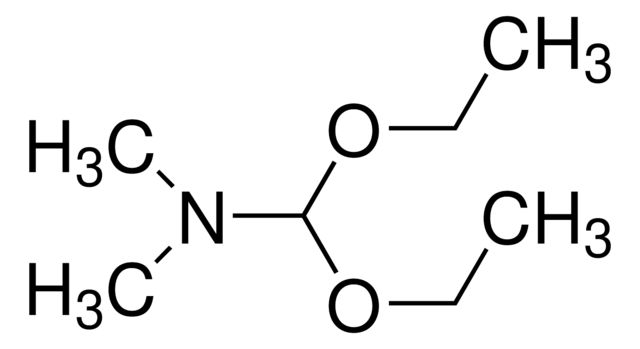
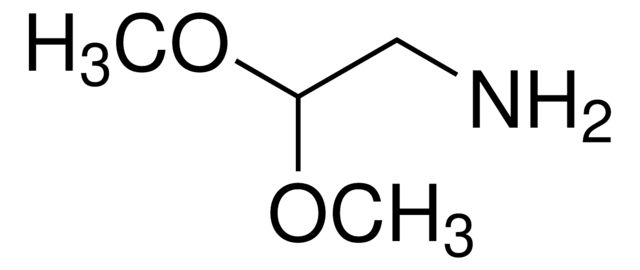
Aminoacetaldehyde dimethyl acetal
99%
Manufacturer: Sigma Aldrich
CAS Number: 22483-09-6
Synonym(S): 2,2-Dimethoxyethylamine
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 25 ML | 121967-25-ML | In Stock | ₹ 3,972.78 |
| 100 ML | 121967-100-ML | In Stock | ₹ 10,110.55 |
| 500 ML | 121967-500-ML | In Stock | ₹ 31,511.58 |
121967 - 25 ML
In Stock
Quantity
1
Base Price: ₹ 3,972.78
GST (18%): ₹ 715.10
Total Price: ₹ 4,687.88
Quality Level
200
Assay
99%
refractive index
n20/D 1.417 (lit.)
bp
135-139 °C/95 mmHg (lit.)
density
0.965 g/mL at 25 °C (lit.)
SMILES string
COC(CN)OC
InChI
1S/C4H11NO2/c1-6-4(3-5)7-2/h4H,3,5H2,1-2H3
InChI key
QKWWDTYDYOFRJL-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
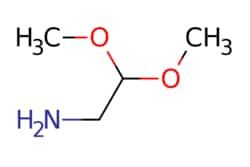 | eMolecules Aminoacetaldehyde dimethyl acetal | 22483-09-6 | MFCD00008135 | 50g | eMolecules | ₹ 4,062.85 | |
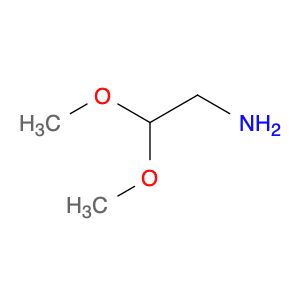 | 2,2-Dimethoxyethylamine | Aaron Chemicals LLC | ₹ 356.00 - ₹ 23,496.00 | |
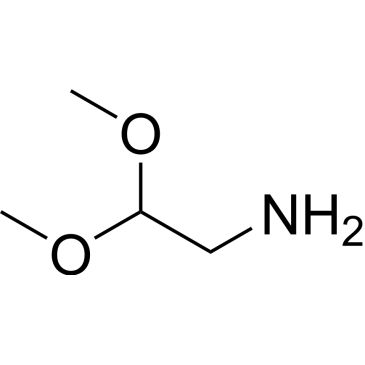 | 2,2-Dimethoxyethanamine | ChemScene | ₹ 1,246.00 - ₹ 13,439.00 | |
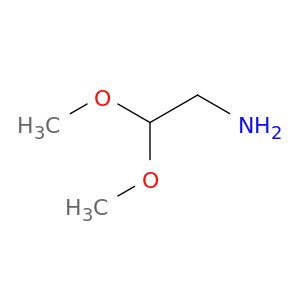 | 22483-09-6 | Aminoacetaldehyde dimethyl acetal | A2B Chem | ₹ 623.00 - ₹ 9,345.00 |
Related Products
Description
- General description: Aminoacetaldehyde dimethyl acetal reacts with sulfone, followed by hydrolysis and reductive amination by adding desired piperazine derivative to yield piperazine derivatives of 2-furanyl[1,2,4]triazolo[1,5-a][1,3,5]triazine[1].Aminoacetaldehyde dimethyl acetal is used as a building block for the synthesis of various acylated and sufonylated oxyenamides.[2] [3]
- Application: Aminoacetaldehyde dimethyl acetal was used in a study to develop a fluorescent substrate for aldehyde dehydrogenase[4]. It was used in preparation of chitosan-dendrimer hybrids having various functional groups such as carboxyl, ester and poly(ethylene glycol)[5]. It was used in an efficient 3-step synthesis of a bicyclic proline analog from L-ascorbic acid[6] and in 3-component reaction catalyzed by MgClO4 leading to α-aminophosphonates[7].
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P210 - P233 - P240 - P280 - P303 + P361 + P353 - P305 + P351 + P338
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
WGK
WGK 2
Flash Point(F)
111.2 °F
Flash Point(C)
44 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves