513830
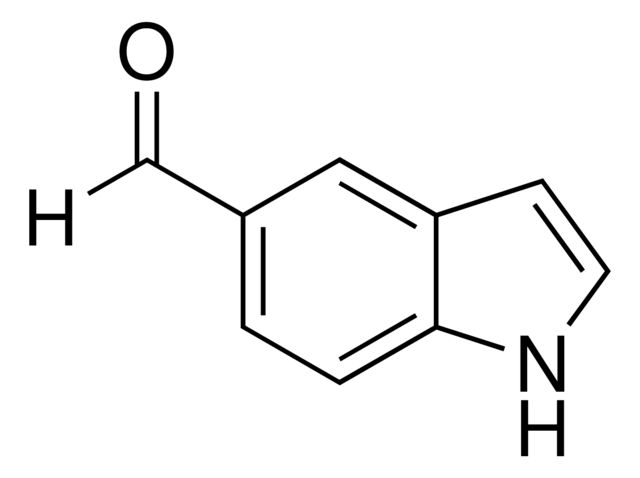
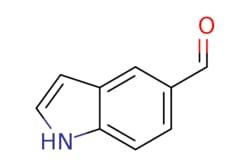
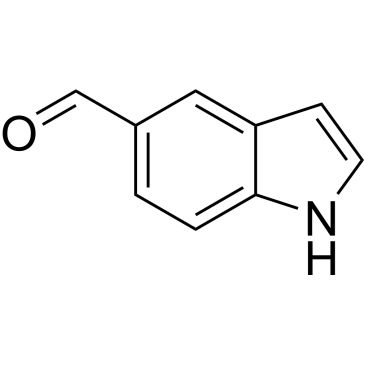
Indole-5-carboxaldehyde
98%
Manufacturer: Sigma Aldrich
CAS Number: 1196-69-6
Synonym(S): 5-Formylindole
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 G | 513830-5-G | In Stock | ₹ 4,633.10 |
513830 - 5 G
In Stock
Quantity
1
Base Price: ₹ 4,633.10
GST (18%): ₹ 833.958
Total Price: ₹ 5,467.058
Quality Level
100
Assay
98%
mp
100-103 °C (lit.)
SMILES string
O=Cc1ccc2[nH]ccc2c1
InChI
1S/C9H7NO/c11-6-7-1-2-9-8(5-7)3-4-10-9/h1-6,10H
InChI key
ADZUEEUKBYCSEY-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Chem-Impex International, Inc. Indole-5-carboxaldehyde | 1196-69-6 | MFCD02093664 | 25G | Chem-Impex International, Inc. | ₹ 16,546.88 | |
 | Chem-Impex International, Inc. Indole-5-carboxaldehyde | 1196-69-6 | MFCD02093664 | 100G | Chem-Impex International, Inc. | ₹ 53,124.99 | |
 | eMolecules Ambeed / Indole-5-carboxaldehyde / 1g / 503281355 / A109501 / / 1196-69-6 / MFCD02093664 / 145.161 / C9H7NO | eMolecules | ₹ 2,695.81 | |
 | 1H-indole-5-carbaldehyde | Aaron Chemicals LLC | ₹ 356.00 - ₹ 1,22,642.00 | |
 | Indole-5-aldehyde | ChemScene | ₹ 1,068.00 - ₹ 1,27,626.00 |
Related Products
Description
- Application: Indole-5-carboxaldehyde can be used as a reactant in the:Preparation of curcumin derivatives as anti-proliferative & anti-inflammatory agents[1]Preparation of analogs of botulinum neurotoxin serotype A protease inhibitors[2]Stereoselective synthesis of dibenzylideneacetone derivatives as β-amyloid imaging probes[3]Synthesis of para-para stilbenophanes by McMurry coupling[4]Stereoselective synthesis of heteroaromatic (E)-α,β-unsaturated ketones from aldehydes[5]Structure-based drug design of aurora kinase A inhibitors[6]Preparation of 5-indolyl linked 15- and 18-membered azacrown ethers to study their cation-π interactions.[7]Preparation of bibenzimidazole derivatives substituted 5-indolyl moiety in the study of inhibition of topoisomerase I activity.[8]Synthesis of (5-(4-(3,4,5-trimethoxybenzoyl)-1H-imidazol-2-yl)-1H-indol-2-yl)(3,4,5-trimethoxyphenyl)methanone[9] and radioiodinated indolochalcone.[10]
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P261 - P264 - P271 - P280 - P302 + P352 - P305 + P351 + P338
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves