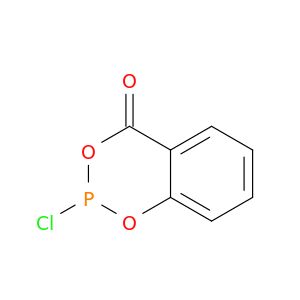
2-Chloro-1,3,2-benzodioxaphosphorin-4-one
95%
Manufacturer: Sigma Aldrich
CAS Number: 5381-99-7
Synonym(S): SalPCl, Salicyl chlorophosphite
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 5 G | 324124-5-G | In Stock | ₹ 2,820.00 |
| 25 G | 324124-25-G | In Stock | ₹ 12,890.00 |
324124 - 5 G
In Stock
Quantity
1
Base Price: ₹ 2,820.00
GST (18%): ₹ 507.60
Total Price: ₹ 3,327.60
Quality Level
200
Assay
95%
form
solid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: ligand
bp
127-128 °C/11 mmHg (lit.)
mp
36-40 °C (lit.)
functional group
phosphine
storage temp.
2-8°C
SMILES string
ClP1OC(=O)c2ccccc2O1
InChI
1S/C7H4ClO3P/c8-12-10-6-4-2-1-3-5(6)7(9)11-12/h1-4H
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences 2-Chloro-1,3,2-benzodioxaphosphorin-4-one 95% | 5381-99-7 | MFCD00013353 | 25G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 38,594.85 | |
 | Sigma Aldrich Fine Chemicals Biosciences 2-Chloro-1,3,2-benzodioxaphosphorin-4-one 95% | 5381-99-7 | MFCD00013353 | 5G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 14,282.72 | |
 | 5381-99-7 | 2-Chloro-4H-benzo[d][1,3,2]dioxaphosphinin-4-one | A2B Chem | -- |
Description
- Application: 2-Chloro-1,3,2-benzodioxaphosphorin-4-one is used as a reagent: In the phosphorylation and phosphitylation of alcohols.[1][2] In the formation of H-phosphonates which are commonly utilized in the synthesis of nucleotides.[1][2] To synthesize nucleoside triphosphates by treating with acyl protected nucleoside.[3]
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P260 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338 - P363
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
EUH014
WGK
WGK 3
Flash Point(F)
closed cup
Flash Point(C)
closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves