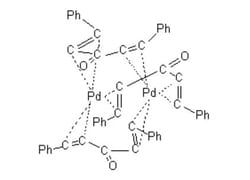
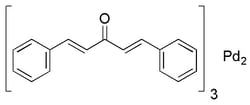
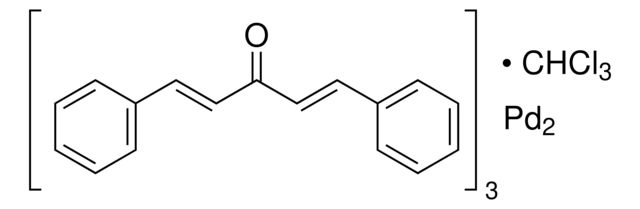
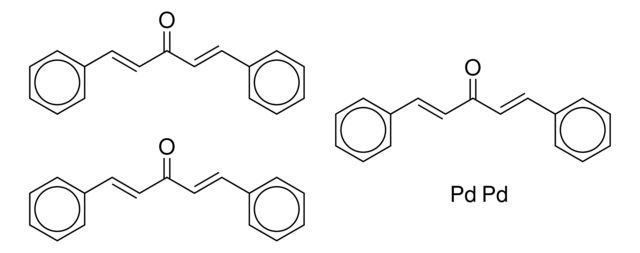
Tris(dibenzylideneacetone)dipalladium(0)
97%
Manufacturer: Sigma Aldrich
CAS Number: 51364-51-3
Synonym(S): Pd2dba3, Pd2(dba)3
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 500 MG | 328774-500-MG | In Stock | ₹ 6,637.80 |
| 1 G | 328774-1-G | In Stock | ₹ 9,435.00 |
| 5 G | 328774-5-G | In Stock | ₹ 44,832.90 |
| 25 G | 328774-25-G | In Stock | ₹ 1,38,161.70 |
| 50 G | 328774-50-G | In Stock | ₹ 2,04,795.00 |
| 100 G | 328774-100-G | In Stock | ₹ 3,94,760.40 |
| 500 G | 328774-500-G | In Stock | ₹ 9,15,383.70 |
328774 - 500 MG
In Stock
Quantity
1
Base Price: ₹ 6,637.80
GST (18%): ₹ 1,194.804
Total Price: ₹ 7,832.604
Quality Level
200
Assay
97%
form
powder
reaction suitability
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Cross Couplingsreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalyst
mp
152-155 °C (lit.)
SMILES string
[Pd].[Pd].O=C(\C=C\c1ccccc1)/C=C/c2ccccc2.O=C(\C=C\c3ccccc3)/C=C/c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;;/h3*1-14H;;/b3*13-11+,14-12+;;
InChI key
CYPYTURSJDMMMP-WVCUSYJESA-N
Other Options
Related Products
Description
- General description: Tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) is a versatile compound widely used as a Pd(0) source in various Pd-mediated transformations.[1] Pd2(dba)3 is known for its high reactivity and ability to facilitate oxidative addition reactions. It is prepared by reacting palladium salts with dibenzylideneacetone ligands. It acts as a catalyst for several reactions including Suzuki cross-coupling, Heck coupling, arylation, Buchwald-Hartwig amination, and fluorination. It is often used in catalytic amounts and has been shown to be effective in promoting coupling reactions between aryl halides and boronic acids.[2]For small scale and high throughput uses, product is also available as ChemBeads (919772)
- Application: Application Guide for Palladium Catalyzed Cross-Coupling ReactionsReactant involved in: Synthesis of azepanes[3] Synthesis of nanosized palladium phosphides upon interaction with white phosphorous Preparation of palladium triphenylphosphine carbonyl cluster complexes Precursor for synthesis of functionalized multiwalled carbon nanotube-palladium complexes used as catalysts for Heck coupling reactions Selective carbon-sulfur bond formation via addition of S-S and S-H bonds to alkynesCatalyst for: Suzuki cross-coupling reactions PCN- and PCS-pincer palladium complex catalyzed tandem allylation Catalyst for Suzuki coupling of aryl chlorides[4] (eq. 1) Catalyst for Heck coupling of aryl chlorides[4] (eq. 2) Catalyst for arylation of ketones[5] (eq. 3) Catalyst for Buchwald-Hartwig amination of aryl halides[6] (eq. 4) Catalyst for fluorination of allylic chlorides[7] (eq. 5) Catalyst for β-arylation of carboxylic esters[8] (eq. 6) Catalyst for carbonylation of 1,1-dichloro-1-alkenes[9] (eq. 7) Catalyst for conversion of aryl and vinyl triflates to aryl and vinyl halides[10] (eq. 8) Pd source for enantioselective Tsuji Allylations[11][12]
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Sens. 1
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves