89231
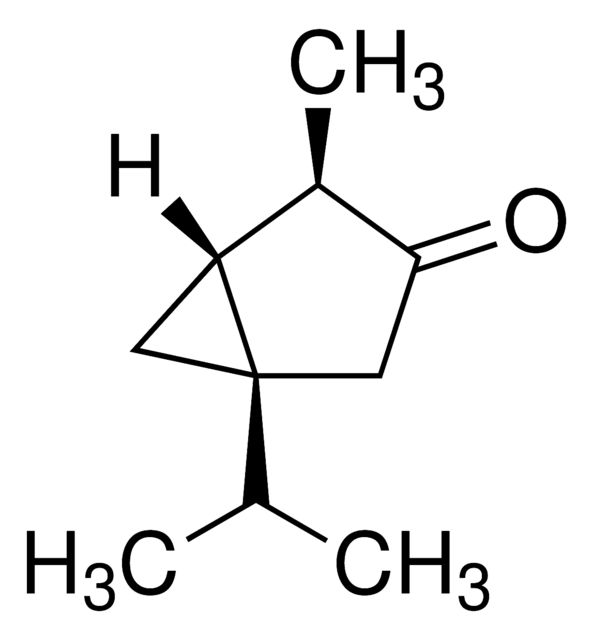
(−)-α-Thujone
≥96.0% (GC)
Manufacturer: Sigma Aldrich
Synonym(S): (-)-alpha-Thujone, (1S,4R)-1-Isopropyl-4-methylbicyclo[3.1.0]hexan-3-one
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 ML | 89231-1-ML | In Stock | ₹ 8,746.60 |
| 5 ML | 89231-5-ML | In Stock | ₹ 27,484.68 |
89231 - 1 ML
In Stock
Quantity
1
Base Price: ₹ 8,746.60
GST (18%): ₹ 1,574.388
Total Price: ₹ 10,320.988
Quality Level
100
Assay
≥96.0% (GC)
form
liquid
optical activity
[α]20/D −19.0±2.0°, neat
refractive index
n20/D 1.450
density
0.914 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2
InChI
1S/C10H16O/c1-6(2)10-4-8(10)7(3)9(11)5-10/h6-8H,4-5H2,1-3H3/t7-,8-,10+/m1/s1
InChI key
USMNOWBWPHYOEA-MRTMQBJTSA-N
Related Products
Description
- General description: (-)-α-Thujone, a monoterpene ketone found in the essential oil of Thuja occidentalis, shows potent antitumorigenic effect.[1]
- Application: A six-step total synthesis of α-thujone and d6-α-thujone, enabling facile access to isotopically labelled metabolites: This study presents a concise method for synthesizing α-thujone, useful for creating isotopically labeled derivatives for research purposes (Thamm et al., 2016). Enhancement of CD3AK cell proliferation and killing ability by α-thujone: Investigates α-thujone′s ability to enhance the proliferation and cytotoxic activity of CD3AK cells, suggesting potential immunological applications (Zhou et al., 2016). α-Thujone exhibits an antifungal activity against F. graminearum by inducing oxidative stress, apoptosis, epigenetics alterations and reduced toxin synthesis: Demonstrates the antifungal effects of α-thujone against Fusarium graminearum, which could make it a valuable alternative to traditional fungicides (Teker et al., 2021).
- Biochem/physiol Actions: GABAA receptor antagonist.
- Other Notes: Chiral building block[2][3][4]
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
WGK
WGK 1
Flash Point(F)
closed cup
Flash Point(C)
closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
Quality Level: 100
Assay: ≥96.0% (GC)
form: liquid
optical activity: [α]20/D −19.0±2.0°, neat
refractive index: n20/D 1.450
density: 0.914 g/mL at 20 °C (lit.)
storage temp.: 2-8°C
SMILES string: CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2
InChI: 1S/C10H16O/c1-6(2)10-4-8(10)7(3)9(11)5-10/h6-8H,4-5H2,1-3H3/t7-,8-,10+/m1/s1
InChI key: USMNOWBWPHYOEA-MRTMQBJTSA-N
Quality Level:
100
Assay:
≥96.0% (GC)
form:
liquid
optical activity:
[α]20/D −19.0±2.0°, neat
refractive index:
n20/D 1.450
density:
0.914 g/mL at 20 °C (lit.)
storage temp.:
2-8°C
SMILES string:
CC(C)[C@@]12C[C@@H]1[C@@H](C)C(=O)C2
InChI:
1S/C10H16O/c1-6(2)10-4-8(10)7(3)9(11)5-10/h6-8H,4-5H2,1-3H3/t7-,8-,10+/m1/s1
InChI key:
USMNOWBWPHYOEA-MRTMQBJTSA-N
Quality Level: 200
Assay: ≥99.0% (HPLC)
form: powder
optical activity: [α]20/D +19±1°, c = 1% in H2O
refractive index: __
density: __
storage temp.: __
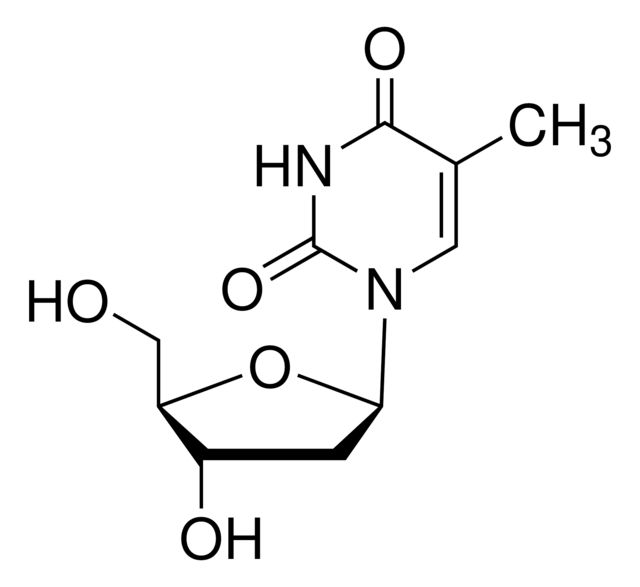
SMILES string: CC1=CN([C@H]2C[C@H](O)[C@@H](CO)O2)C(=O)NC1=O
InChI: 1S/C10H14N2O5/c1-5-3-12(10(16)11-9(5)15)8-2-6(14)7(4-13)17-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,16)/t6-,7+,8+/m0/s1
InChI key: IQFYYKKMVGJFEH-XLPZGREQSA-N
Quality Level:
200
Assay:
≥99.0% (HPLC)
form:
powder
optical activity:
[α]20/D +19±1°, c = 1% in H2O
refractive index:
__
density:
__
storage temp.:
__
SMILES string:
CC1=CN([C@H]2C[C@H](O)[C@@H](CO)O2)C(=O)NC1=O
InChI:
1S/C10H14N2O5/c1-5-3-12(10(16)11-9(5)15)8-2-6(14)7(4-13)17-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,16)/t6-,7+,8+/m0/s1
InChI key:
IQFYYKKMVGJFEH-XLPZGREQSA-N