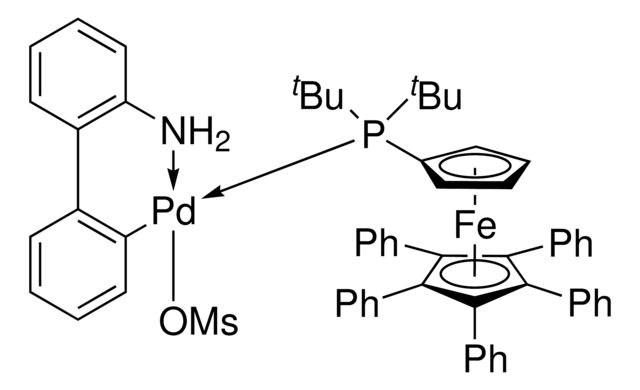
QPhos Pd G3
Manufacturer: Sigma Aldrich
CAS Number: 2021255-91-2
Synonym(S): QPhos G3 Palladacycle, QPhos Palladacycle, [2′-(Amino-κN)[1,1′-biphenyl]-2-yl-κC][1′-[bis(1,1-dimethylethyl)phosphino]-1,2,3,4,5-pentaphenylferrocene](methanesulfonato-κO)palladium
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 250 MG | 903027-250-MG | In Stock | ₹ 17,893.20 |
| 1 G | 903027-1-G | In Stock | ₹ 50,616.00 |
903027 - 250 MG
In Stock
Quantity
1
Base Price: ₹ 17,893.20
GST (18%): ₹ 3,220.776
Total Price: ₹ 21,113.976
form
powder
feature
generation 3
reaction suitability
core: palladiumreaction type: Buchwald-Hartwig Cross Coupling Reactionreaction type: Heck Reactionreaction type: Hiyama Couplingreaction type: Negishi Couplingreaction type: Sonogashira Couplingreaction type: Stille Couplingreaction type: Suzuki-Miyaura Couplingreagent type: catalystreaction type: Cross Couplings
functional group
phosphine
SMILES string
O=S(=O)([O-][Pd+2]1([C-]=2C=CC=CC2C=3C=CC=CC3[NH2]1)[P](C(C)(C)C)(C(C)(C)C)[C-]45[CH]6=[CH]7[CH]8=[CH]4[Fe+2]7869%10%11%125C=%13(C=%14C=CC=CC%14)C%12(C=%15C=CC=CC%15)=C%11(C=%16C=CC=CC%16)[C-]%10(C=%17C=CC=CC%17)C%139C=%18C=CC=CC%18)C
InChI
InChI=1S/C35H25.C13H22P.C12H10N.CH4O3S.Fe.Pd/c1-6-16-26(17-7-1)31-32(27-18-8-2-9-19-27)34(29-22-12-4-13-23-29)35(30-24-14-5-15-25-30)33(31)28-20-10-3-11-21-28;1-12(2,3)14(13(4,5)6)11-9-7-8-10-11;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;;/h1-25H;7-10H,1-6H3;1-6,8-9H,13H2;1H3,(H,2,3,4);;/q3*-1;;2*+2/p-1
InChI key
NNVAEYGNFLUPRU-UHFFFAOYSA-M
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences QPhos Pd G3 | 2021255-91-2 | MFCD12911997 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 68,052.96 | |
 | 2021255-91-2 | QPHOS PD G3 | A2B Chem | ₹ 4,539.00 - ₹ 26,789.00 |
Related Products
Description
- General description: QPhos Pd G3 is a third generation (G3) Buchwald precatalyst. It is air, moisture and thermally-stable and is highly soluble in a wide range of common organic solvents. It has long life in solutions. QPhos Pd G3 is an excellent reagent for palladium catalyzed cross-coupling reactions. Some of its unique features include lower catalyst loadings, shorter reaction time, efficient formation of the active catalytic species and accurate control of ligand: palladium ratio.
- Application: QPhos Pd G3 has been used as a precatalyst for the arylboration of isoprene with regioselectivity. It may also be used for a variety of aminination and etherifications of aryl halides.
- Other Notes: Technology Spotlight: G3 and G4 Buchwald PrecatalystsRegioselective Arylboration of Isoprene and Its Derivatives by Pd/Cu Cooperative CatalysisSynthesis of Complex Drug like Molecules by the Use of Highly Functionalized Bench-Stable Organozinc Reagents
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable