901991
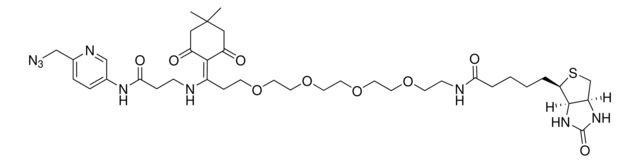
Dde biotin picolyl azide
Manufacturer: Sigma Aldrich
CAS Number: 2055048-42-3
Synonym(S): N-[6-(Azidomethyl)-3-pyridinyl]-5-(4,4-dimethyl-2,6-dioxocyclohexylidene)-25-[(3aS,4S,6aR)-hexahydro-2-oxo-1H-thieno[3,4-d]imidazol-4-yl]-21-oxo-8,11,14,17-tetraoxa-4,20-diazapentacosanamide
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 MG | 901991-10-MG | In Stock | ₹ 33,828.13 |
901991 - 10 MG
In Stock
Quantity
1
Base Price: ₹ 33,828.13
GST (18%): ₹ 6,089.063
Total Price: ₹ 39,917.193
form
solid
reaction suitability
reaction type: click chemistry
storage temp.
−20°C
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | eMolecules Broadpharm / Dde Biotin-PEG4-Picolyl azide / 5mg / 340377095 / BP-23340 / 95.000 / 2055048-42-3 / MFCD30458027 / 815.990 / C38H57N9O9S | eMolecules | ₹ 37,598.94 | |
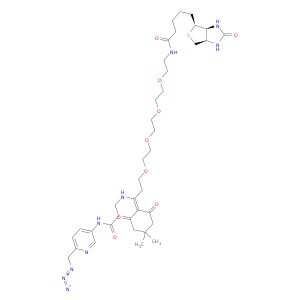 | Dde Biotin-PEG4-Picolyl azide | Aaron Chemicals LLC | -- | |
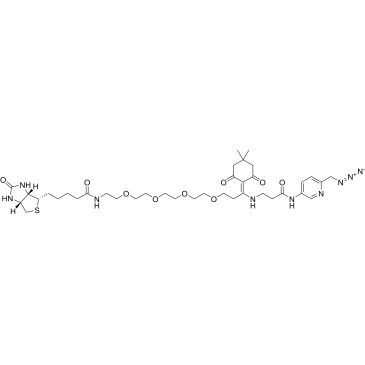 | Dde Biotin-PEG4-Picolyl azide | ChemScene | ₹ 2,64,152.00 | |
 | 2055048-42-3 | Dde Biotin-PEG4-Picolyl azide | A2B Chem | ₹ 16,465.00 - ₹ 3,70,240.00 |
Related Products
Description
- Application: Dde biotin picolyl azide is a unique alkyne-reactive, cleavable biotin probe that allows for release of the captured biomolecules from streptavidin under mild conditions. This reagent contains a biotin moiety linked to a picolyl azide moiety through a spacer arm containing a hydrazine-cleavable Dde moiety. Captured biomolecules can be efficiently released, typically >90% with 2% hydrazine in aqueous media.Dde biotin picolyl azide incorporates a copper-chelating motif that dramatically accelerates the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction under conditions relevant to biomolecular labeling. Discovery of azides with an internal Cu(I)-chelating motif greatly enhances the utility of the CuAAC reaction for various applications. Some examples are monitoring the dynamic glycan biosynthesis in living organisms; sensitive detection of metabolically-labeled proteins, DNAs, and RNAs; and many other applications where rate acceleration and reduced cell toxicity are highly desirable.
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable