915572
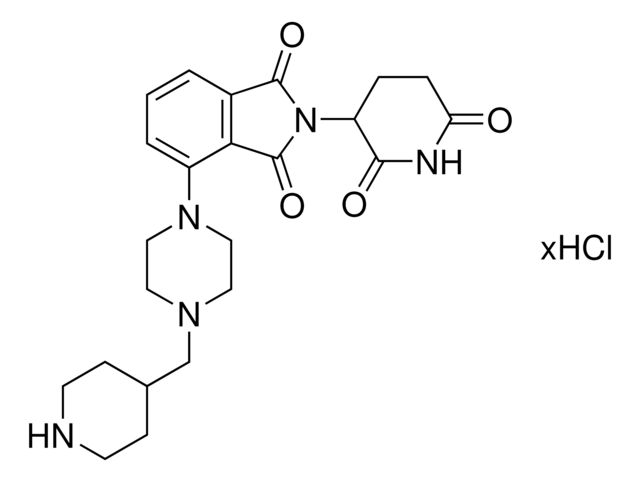
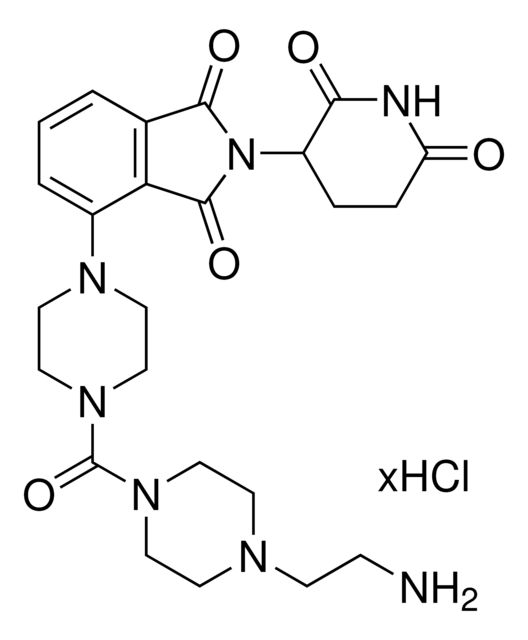
Pomalidomide-dipiperazine-NH2 hydrochloride
≥95%
Manufacturer: Sigma Aldrich
Synonym(S): 4-(4-(4-(2-Aminoethyl)piperazine-1-carbonyl)piperazin-1-yl)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride, Crosslinker−E3 ligase ligand conjugate, Pomalidomide conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 50 MG | 915572-50-MG | In Stock | ₹ 45,154.80 |
915572 - 50 MG
In Stock
Quantity
1
Base Price: ₹ 45,154.80
GST (18%): ₹ 8,127.864
Total Price: ₹ 53,282.664
ligand
pomalidomide
Quality Level
100
Assay
≥95%
form
powder
reaction suitability
reactivity: carboxyl reactivereagent type: ligand-linker conjugate
functional group
amine
storage temp.
2-8°C
SMILES string
O=C(N(C1CCC(NC1=O)=O)C2=O)C3=C2C=CC=C3N4CCN(C(N5CCN(CCN)CC5)=O)CC4
Related Products
Description
- Application: Protein degrader building block Pomalidomide-dipiperazine-NH2 hydrochloride enables the synthesis of molecules for targeted protein degradation and PROTAC (proteolysis-targeting chimeras) technology. This conjugate contains a Cereblon (CRBN)-recruiting ligand, a rigid linker, and a pendant amine for reactivity with an acid on the target warhead. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and PROTAC, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate PROTAC libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
- Other Notes: Technology Spotlight: Degrader Building Blocks for Targeted Protein DegradationPortal: Building PROTAC® Degraders for Targeted Protein DegradationTargeted Protein Degradation by Small MoleculesSmall-Molecule PROTACS: New Approaches to Protein DegradationTargeted Protein Degradation: from Chemical Biology to Drug Discovery
- Legal Information: PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable