QBD10503
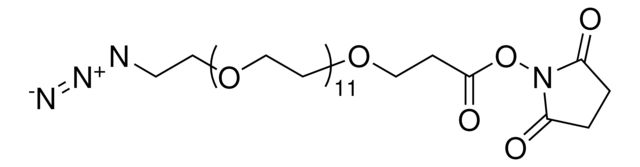
Azido-dPEG®8-NHS ester
Manufacturer: Sigma Aldrich
CAS Number: 1204834-00-3
Synonym(S): 2,5-Dioxo-1-pyrrolidinyl 3-[(23-azido-3,6,9,12,15,18,21-heptaoxatricos-1-yl)oxy]propanoate, Polyethylene glycol
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 100 MG | QBD10503-100-MG | In Stock | ₹ 19,766.45 |
| 1000 MG | QBD10503-1000-MG | In Stock | ₹ 64,224.73 |
QBD10503 - 100 MG
In Stock
Quantity
1
Base Price: ₹ 19,766.45
GST (18%): ₹ 3,557.961
Total Price: ₹ 23,324.411
Assay
>90%
form
solid or viscous liquid
reaction suitability
reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group
NHS esterazide
polymer architecture
shape: linearfunctionality: heterobifunctional
shipped in
ambient
storage temp.
−20°C
SMILES string
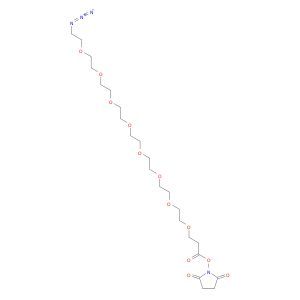
O=C(ON1C(CCC1=O)=O)CCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-]
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | eMolecules Azido-PEG8-NHS ester | 1204834-00-3 | MFCD13184949 | 100mg | eMolecules | ₹ 32,752.00 | |
 | Sigma Aldrich Fine Chemicals Biosciences Azido-dPEG(R)8-NHS ester | Purity: >90% | Mol Wt: 564.58 | 1204834-00-3 | MFCD13184949 | 100MG | Sigma Aldrich Fine Chemicals Biosciences | ₹ 32,271.40 | |
 | Azido-PEG8-NHS ester | ChemScene | ₹ 9,790.00 - ₹ 52,688.00 | |
 | 1204834-00-3 | 1-Azido-3,6,9,12,15,18,21,24-octaoxaheptacosan-27-oic acid succinimidyl ester | A2B Chem | ₹ 13,617.00 - ₹ 1,14,098.00 |
Description
- Features and Benefits: The azido-dPEG®8-NHS ester contains an azide function on one end of a single molecular weight dPEG® spacer(32.2 Å) and a reactive group (NHS ester) on the other end of the spacer. The dPEG® spacer is hydrophilic and non-immunogenic and improves the water solubility of the target molecule while reducing the immunogenicity and increasing the hydrodynamic volume of the target. N-hydroxysuccinimide (NHS) ester readily react with amines in aqueous solution or organic solvent. The azide group reacts with an alkyne in the well-known click chemistry reaction. The click chemistry reaction proceeds by copper(I) or ruthenium catalysis or in a strain-catalyzed reaction with certain types of alkyne partners.
- Legal Information: Products Protected under U.S. Patents # 7,888,536 B2
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Assay: >90%
form: solid or viscous liquid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group: NHS esterazide
polymer architecture: shape: linearfunctionality: heterobifunctional
shipped in: ambient
storage temp.: −20°C
SMILES string: O=C(ON1C(CCC1=O)=O)CCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-]
Assay:
>90%
form:
solid or viscous liquid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group:
NHS esterazide
polymer architecture:
shape: linearfunctionality: heterobifunctional
shipped in:
ambient
storage temp.:
−20°C
SMILES string:
O=C(ON1C(CCC1=O)=O)CCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-]
Assay: >90%
form: solid or viscous liquid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
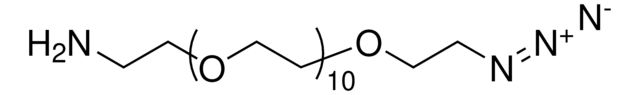
functional group: __
polymer architecture: shape: linearfunctionality: heterobifunctional
shipped in: ambient
storage temp.: −20°C
SMILES string: O=C(CCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-])ON1C(CCC1=O)=O
Assay:
>90%
form:
solid or viscous liquid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group:
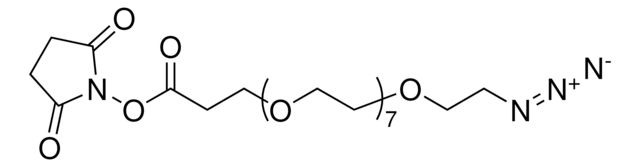
__
polymer architecture:
shape: linearfunctionality: heterobifunctional
shipped in:
ambient
storage temp.:
−20°C
SMILES string:
O=C(CCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-])ON1C(CCC1=O)=O
Assay: >90%
form: solid or viscous liquid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
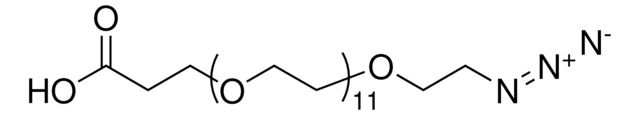
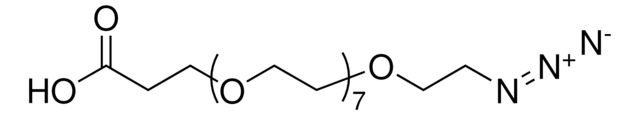
functional group: azidecarboxylic acid
polymer architecture: shape: linearfunctionality: heterobifunctional
shipped in: ambient
storage temp.: −20°C
SMILES string: OC(CCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-])=O
Assay:
>90%
form:
solid or viscous liquid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group:
azidecarboxylic acid
polymer architecture:
shape: linearfunctionality: heterobifunctional
shipped in:
ambient
storage temp.:
−20°C
SMILES string:
OC(CCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-])=O
Assay: >90%
form: solid or viscous liquid
reaction suitability: reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group: __
polymer architecture: shape: linearfunctionality: heterobifunctional
shipped in: ambient
storage temp.: −20°C
SMILES string: OC(CCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-])=O
Assay:
>90%
form:
solid or viscous liquid
reaction suitability:
reaction type: click chemistryreagent type: cross-linking reagentreaction type: click chemistry
functional group:
__
polymer architecture:
shape: linearfunctionality: heterobifunctional
shipped in:
ambient
storage temp.:
−20°C
SMILES string:
OC(CCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-])=O