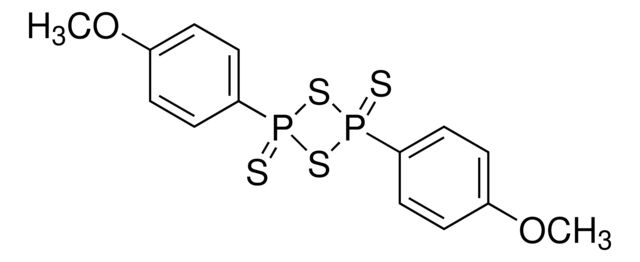
227439
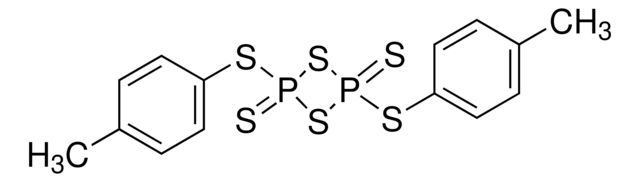
Lawesson reagent
97%
Manufacturer: Sigma Aldrich
CAS Number: 19172-47-5
Synonym(S): 2,4-Bis(4-methoxyphenyl)-2,4-dithioxo-1,3,2,4-dithiadiphosphetane, 2,4-Bis-(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane 2,4-disulfide, 4-Methoxyphenylthiophosphoric cyclic di(thioanhydride), LR
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 10 G | 227439-10-G | In Stock | ₹ 2,305.73 |
| 25 G | 227439-25-G | In Stock | ₹ 3,052.65 |
| 100 G | 227439-100-G | In Stock | ₹ 9,796.63 |
227439 - 10 G
In Stock
Quantity
1
Base Price: ₹ 2,305.73
GST (18%): ₹ 415.031
Total Price: ₹ 2,720.761
Quality Level
100
Assay
97%
form
powder
mp
228-230 °C (lit.)
SMILES string
COc1ccc(cc1)P2(=S)SP(=S)(S2)c3ccc(OC)cc3
InChI
1S/C14H14O2P2S4/c1-15-11-3-7-13(8-4-11)17(19)21-18(20,22-17)14-9-5-12(16-2)6-10-14/h3-10H,1-2H3
InChI key
CFHGBZLNZZVTAY-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
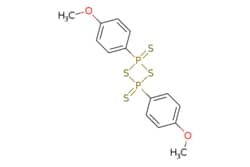 | eMolecules Lawesson's reagent | 19172-47-5 | MFCD00005171 | 25g | eMolecules | ₹ 4,426.86 |
Related Products
Description
- General description: Lawesson′s reagent is generally used as a thiation agent in organic synthesis for the conversion of oxygen functionalities into their thio analogs. It facilitates the conversion of the carbonyl group to thiocarbonyl group as well as carbon-oxygen single bond into a carbon-sulfur single bond.[1][2][2]
- Application: Lawesson reagent can be used as a reagent to synthesize:Oxthiaphosphinine-3-sulfide derivatives by the reaction with Mannich bases of β-naphthol and 8-hydroxyquinoline.[3]1,3,5,2-Trithiaphosphinane-2-sulfide derivatives by reacting with benzaldehyde in the presence of trialkyl phosphite.[3]2,4,6-Triphenyl-1,3,5-trithiane from benzaldehyde and ethyl acrylate.[3]9-Benzanthronethione by thionation of 9-benzanthone oxime.[2]1,2,4-Trithiolane from 2,2,4,4-tetramethyl-3-thioxocyclobutanone S-oxide.[4]Sulfur derivatives of triterpenic oxo compounds.[5]Tropothione in situ at room temperature and to trap it with dieneophiles.[6]
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Water-react 2
Supplementary Hazards
EUH029
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable