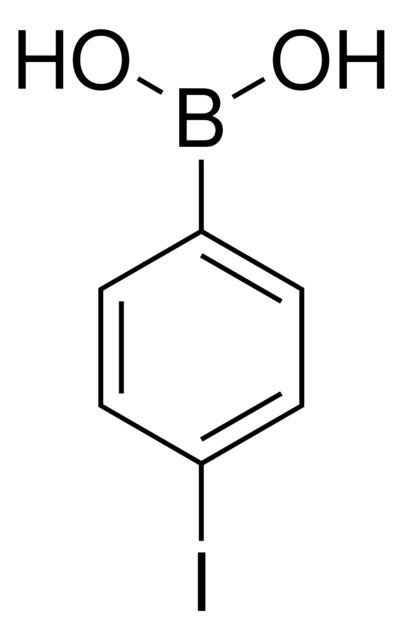
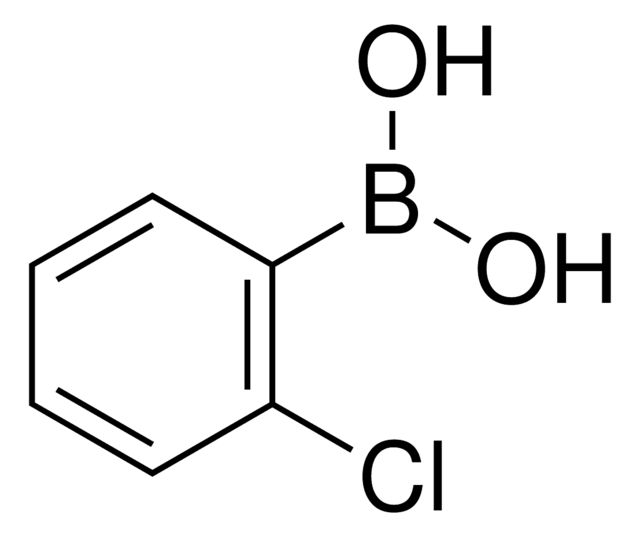
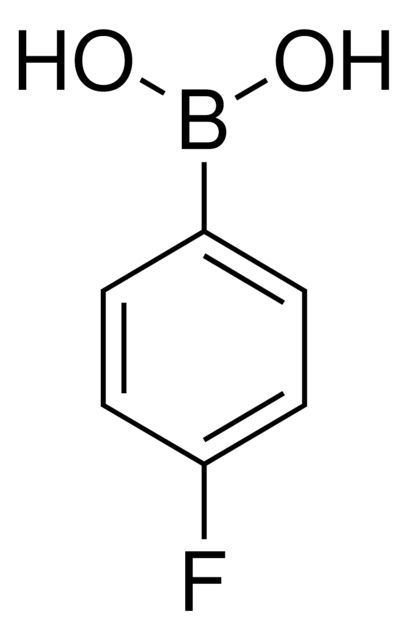
4-Fluorophenylboronic acid
≥95%
Manufacturer: Sigma Aldrich
CAS Number: 1765-93-1
Synonym(S): (4-Fluorophenyl)boric acid, (4-Fluorophenyl)dihydroxyborane, (4-Fluorophenyl)dihydroxyboron, (p-Fluorophenyl)boric acid, 4-Fluorobenzeneboronic acid, p-Fluorobenzylboronic acid, p-Fluorophenylboronic acid, NSC 142683
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 417556-1-G | In Stock | ₹ 1,926.85 |
| 5 G | 417556-5-G | In Stock | ₹ 8,259.48 |
| 25 G | 417556-25-G | In Stock | ₹ 18,608.18 |
417556 - 1 G
In Stock
Quantity
1
Base Price: ₹ 1,926.85
GST (18%): ₹ 346.833
Total Price: ₹ 2,273.683
Quality Level
100
Assay
≥95%
form
powder
mp
262-265 °C (lit.)
SMILES string
OB(O)c1ccc(F)cc1
InChI
1S/C6H6BFO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
LBUNNMJLXWQQBY-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
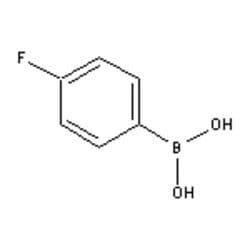 | Accela Chembio Inc 4-fluorophenylboronic Acid | 100g | 1765-93-1 | MFCD00039136 | 97+% | Shelf Life: 1440 Days | Light Sensitive/nitrogen Or Argon/+4 | Accela Chembio Inc | ₹ 3,034.90 | |
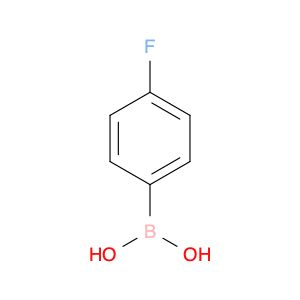 | Boronic acid, B-(4-fluorophenyl)- | Aaron Chemicals LLC | ₹ 356.00 - ₹ 14,952.00 | |
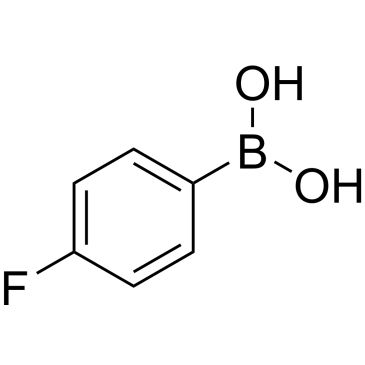 | 4-Fluorobenzeneboronic acid | ChemScene | ₹ 623.00 - ₹ 20,203.00 | |
 | 1765-93-1 | 4-Fluorophenylboronic acid | A2B Chem | ₹ 445.00 - ₹ 10,057.00 |
Related Products
Description
- Application: 4-Fluorophenylboronic acid can be used as a reactant in coupling reactions with arenediazonium tetrafluoroborates,[1] iodonium salts, and iodanes.[2] It is also used to make novel biologically active terphenyls.[3]It can also be used as a reactant in: Suzuki coupling using microwave and triton B catalyst.[4] Pd-catalyzed direct arylation of pyrazoles with phenylboronic acids.[5] Mizoroki-Heck and Suzuki-Miyaura coupling reactions catalyzed by palladium nanoparticles.[6] Cu-catalyzed Petasis reactions.[7] Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence.[8] Ruthenium catalyzed direct arylation.[9] Rh-catalyzed asymmetric conjugate additions.[10] Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.[11] Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.[12] Suzuki cross-coupling of tetrabromothiophene.[13]Palladium-catalyzed addition to nitriles.[14]
- Other Notes: Contains varying amounts of anhydride
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P261 - P264 - P271 - P301 + P312 - P302 + P352 - P305 + P351 + P338
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves