439320
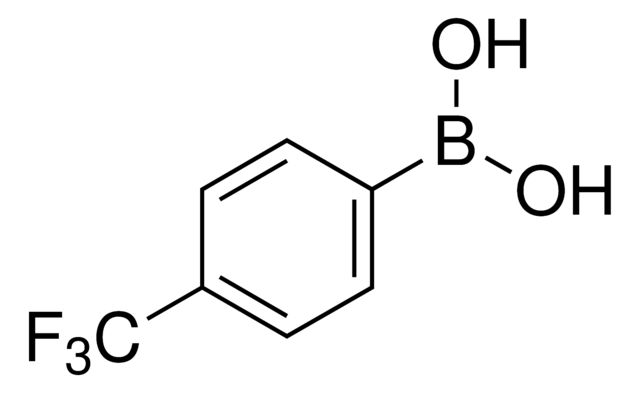
4-(Trifluoromethyl)phenylboronic acid
≥95.0%
Manufacturer: Sigma Aldrich
CAS Number: 128796-39-4
Synonym(S): α,α,α-Trifluoro-p-tolylboronic acid, 4-(Trifluoromethyl)benzeneboronic acid, [p-(Trifluoromethyl)phenyl]boronic acid
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 439320-1-G | In Stock | ₹ 4,633.10 |
| 5 G | 439320-5-G | In Stock | ₹ 10,316.23 |
439320 - 1 G
In Stock
Quantity
1
Base Price: ₹ 4,633.10
GST (18%): ₹ 833.958
Total Price: ₹ 5,467.058
Quality Level
100
Assay
≥95.0%
mp
245-250 °C (lit.)
SMILES string
OB(O)c1ccc(cc1)C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-1-3-6(4-2-5)8(12)13/h1-4,12-13H
InChI key
ALMFIOZYDASRRC-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences 4-(Trifluoromethyl)phenylboronic acid >=95.0% | 128796-39-4 | MFCD00151855 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 7,983.30 | |
 | Boronic acid, B-[4-(trifluoromethyl)phenyl]- | Aaron Chemicals LLC | ₹ 356.00 - ₹ 33,731.00 | |
 | 4-Trifluoromethylphenylboronic acid | ChemScene | ₹ 712.00 - ₹ 56,426.00 |
Related Products
Description
- Application: 4-(Trifluoromethyl)phenylboronic acid can be used as a reactant in:Site-selective Suzuki-Miyaura cross-coupling reactions.[1]Palladium-catalyzed direct arylation reactions.[2]Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence.[3]Ruthenium catalyzed direct arylation.[4]Ligand-free copper-catalyzed coupling reactions.[5]Amination and conjugate addition reactions.[6]Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.[7]Rhodium-catalyzed asymmetric 1,4-addition reactions.[8]Copper-catalyzed nitration reactions.[9]Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.[10]Palladium catalyzed allylation reaction with allyl alcohols.[11]N-Arylation of imidazoles and amines in the presence of copper-exchanged fluorapatite as a catalyst.[12]It can also be used as a reactant to prepare:Thiazole derivatives for printable electronics.[13]Terphenyl benzimidazoles as tubulin polymerization inhibitors.[14] Aryl ketones by cross-coupling reaction with acid chlorides.[15]
- Other Notes: Contains varying amounts of anhydride
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves