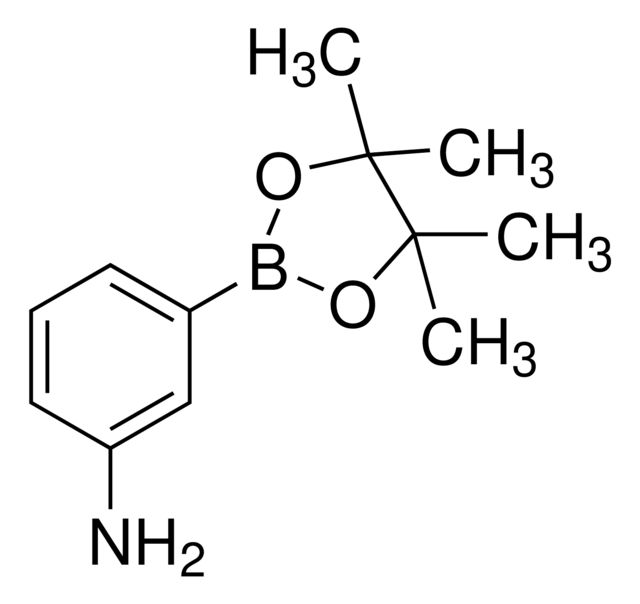
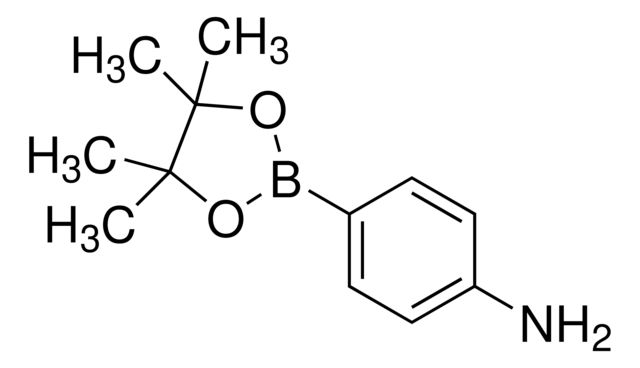
4-Aminophenylboronic acid pinacol ester
97%
Manufacturer: Sigma Aldrich
CAS Number: 214360-73-3
Synonym(S): 2-(4-Aminophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzeneamine, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenylamine, 4-Aminophenylboronic acid, pinacol cyclic ester
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 518751-1-G | In Stock | ₹ 4,784.65 |
| 5 G | 518751-5-G | In Stock | ₹ 20,264.40 |
518751 - 1 G
In Stock
Quantity
1
Base Price: ₹ 4,784.65
GST (18%): ₹ 861.237
Total Price: ₹ 5,645.887
Quality Level
100
Assay
97%
mp
165-169 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(N)cc2
InChI
1S/C12H18BNO2/c1-11(2)12(3,4)16-13(15-11)9-5-7-10(14)8-6-9/h5-8H,14H2,1-4H3
InChI key
ZANPJXNYBVVNSD-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Chemscene AbaChemscene, 4-(4, 4, 5, 5-Tetramethyl-1, 3, 2-dioxaborolan-2-yl)aniline, 214360-73-3, 25g, Formula:C12H18BNO2, M. Wt. :219.09, Purity:>98% | Chemscene | ₹ 3,284.10 | |
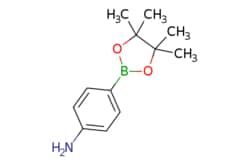 | eMolecules 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline | 214360-73-3 | MFCD02093721 | 100g | eMolecules | ₹ 15,459.30 | |
 | Aobchem 4-Aminophenylboronic acid pinacol ester, AOBCHEM USA 10299-25G. 214360-73-3. MFCD02093721 | Aobchem | ₹ 8,622.32 | |
 | 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline | Aaron Chemicals LLC | ₹ 356.00 - ₹ 41,919.00 | |
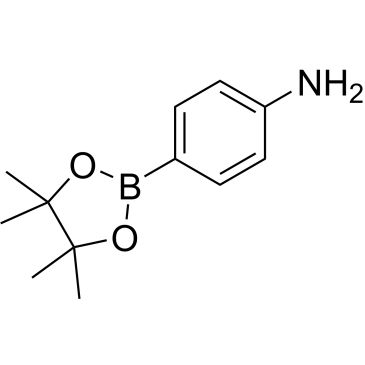 | 4-Aminophenylboronic acid pinacol ester | ChemScene | ₹ 801.00 - ₹ 52,065.00 |
Related Products
Description
- Application: 4-Aminophenylboronic acid pinacol ester can be used as a reagent for:The preparation of substituted 3-phenyl-4H-1-benzopyran-4-ones by reacting with iodochromones via Pd catalyzed Suzuki-Miyaura cross-coupling reaction.[1] Mercury(II) detection by fluorometry with new fluorogenic indicators based on through-bond energy transfer from pentaquinone to rhodamine.[2] Rhodium-catalyzed amination reactions.[3] Palladium-catalyzed Suzuki cross-coupling to synthesize potential antitubercular and antimicrobial compounds.[4] It can also be used to prepare: Hexaphenylbenzene derivatives as a potential bioprobe and multichannel keypad system.[5]Pyromellitic diimide-based polymer as matrix for solution-processable n-channel field-effect transistors.[6] Alternating copolymers of oligoarylenes and naphthalene bisimides as low band-gap semiconductors with electrochemical and spectroelectrochemical behavior.[7] γ-secretase modulators in the treatment of amyloid β formation.[8]
SAFETY INFORMATION
Pictograms
Signal Word
Warning
Hazard Statements
Precautionary Statements
P261 - P264 - P271 - P280 - P302 + P352 - P305 + P351 + P338
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves