594539
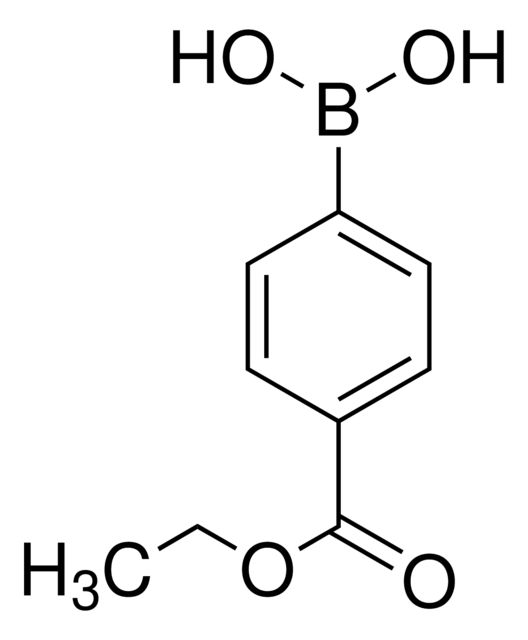
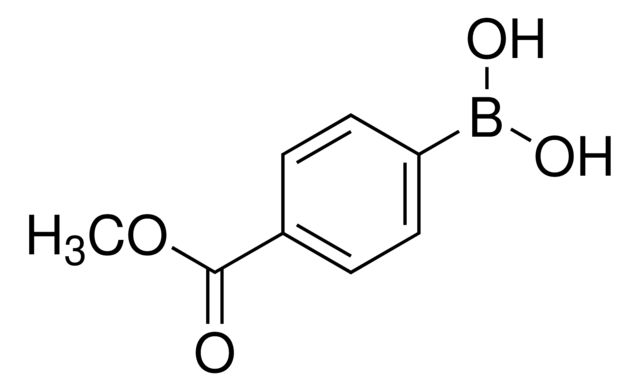
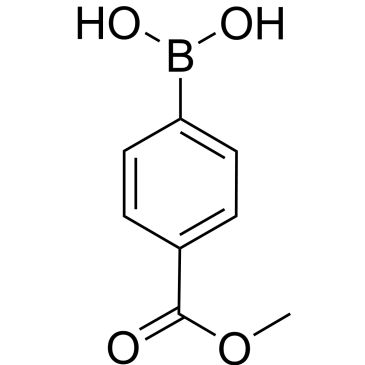
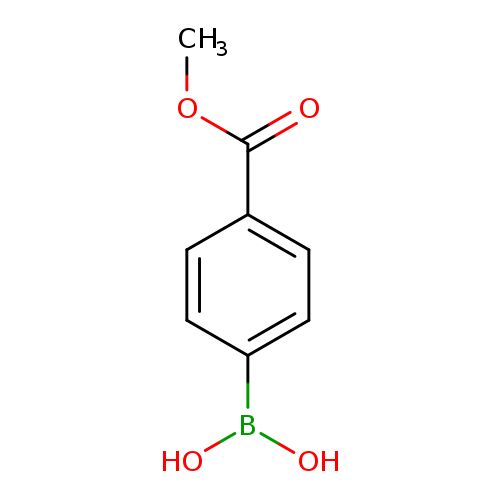
4-Methoxycarbonylphenylboronic acid
≥95%
Manufacturer: Sigma Aldrich
CAS Number: 99768-12-4
Synonym(S): (4-Carbomethoxyphenyl)boronic acid, 4-Carbomethoxybenzeneboronic acid, 4-Methoxycarbonylbenzeneboronic acid, 4-borono-benzoic acid 1-methyl ester, p-(Methoxycarbonyl)boronic acid, p-(Methoxycarbonyl)phenylboronic acid, p-borono-benzoic acid methyl ester, Methyl 4-boronobenzoate, Methyl p-boronobenzoate
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 594539-1-G | In Stock | ₹ 3,160.90 |
| 5 G | 594539-5-G | In Stock | ₹ 9,850.75 |
594539 - 1 G
In Stock
Quantity
1
Base Price: ₹ 3,160.90
GST (18%): ₹ 568.962
Total Price: ₹ 3,729.862
Quality Level
100
Assay
≥95%
form
powder
mp
197-200 °C (lit.)
SMILES string
COC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO4/c1-13-8(10)6-2-4-7(5-3-6)9(11)12/h2-5,11-12H,1H3
InChI key
PQCXFUXRTRESBD-UHFFFAOYSA-N
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Aobchem 4-Methoxycarbonylphenylboronic acid, AOBCHEM USA 10265-100G. 99768-12-4. MFCD01632203 | Aobchem | ₹ 14,922.63 | |
 | Sigma Aldrich Fine Chemicals Biosciences 4-Methoxycarbonylphenylboronic acid >=95% | 99768-12-4 | MFCD01632203 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 5,811.70 | |
 | Sigma Aldrich Fine Chemicals Biosciences 4-Methoxycarbonylphenylboronic acid >=95% | 99768-12-4 | MFCD01632203 | 5G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 14,718.82 | |
 | Methyl 4-Boronobenzoate | Aaron Chemicals LLC | ₹ 267.00 - ₹ 50,819.00 | |
 | Methyl 4-boronobenzoate | ChemScene | ₹ 534.00 - ₹ 49,751.00 | |
 | 99768-12-4 | 4-Methoxycarbonylphenylboronic acid | A2B Chem | ₹ 356.00 - ₹ 1,246.00 |
Related Products
Description
- Application: Reagent used forTandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence[1] Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides[2] One-pot ipso-nitration of arylboronic acids[3] Copper-catalyzed nitration[4] Cyclocondensation followed by palladium-phosphine-catalyzed Suzuki-Miyaura coupling[5] Reagent used in Preparation ofBiaryls via nickel-catalyzed Suzuki-Miyaura cross-coupling reaction of aryl halides with arylboronic acid[6]† Chromenones and their bradykinin B1 antagonistic activit[7]† Pt nanoparticles@Photoactive metal-organic frameworks resulting in efficient hydrogen evolution via synergistic photoexcitation and electron injectio[8]† Salicylate-based thienylbenzoic acids as E. coli methionine aminopeptidase inhibitor[9]†
- Other Notes: Contains varying amount of anhydride
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves