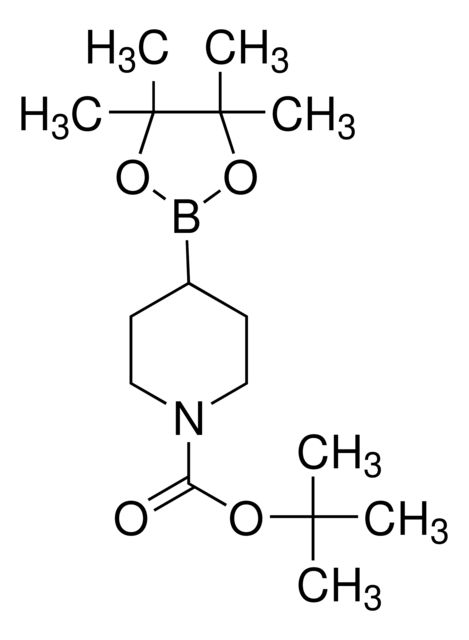
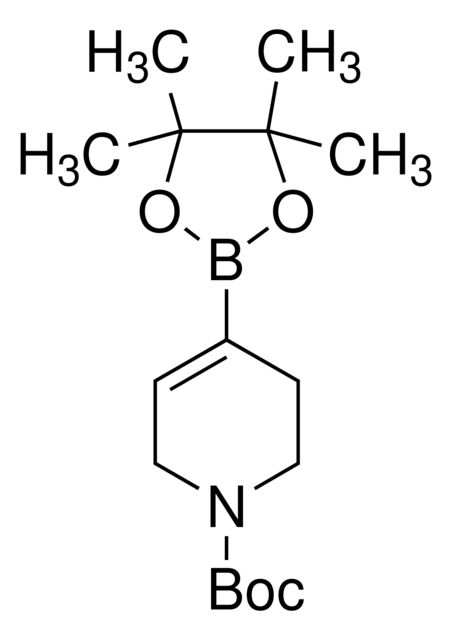
N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
95%
Manufacturer: Sigma Aldrich
CAS Number: 286961-14-6
Synonym(S): (1-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl)-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 706531-1-G | In Stock | ₹ 5,737.25 |
706531 - 1 G
In Stock
Quantity
1
Base Price: ₹ 5,737.25
GST (18%): ₹ 1,032.705
Total Price: ₹ 6,769.955
Quality Level
100
Assay
95%
form
powder
mp
100-114 °C
SMILES string
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
InChI key
VVDCRJGWILREQH-UHFFFAOYSA-N
Other Options
Related Products
Description
- General description: Boc-THP-Bpin is frequently used in metal catalyzed cross-coupling reactions, such as Suzuki-Miyaura and Negishi couplings.[1][2]
- Application: Reagent used for Suzuki-Miyaura cross-coupling using palladium phosphine catalyst[1] Palladium-catalyzed ligand-controlled regioselective Suzuki coupling[2] Palladium-catalyzed Suzuki-Miyaura coupling[3] Suzuki coupling followed by iodolactonization reaction[4] Wrenchnolol derivative optimized for gene activation in cells[5] Reagent used in Preparation of several enzymatic inhibitors and receptor ligandsOrally active anaplastic lymphoma kinase inhibitors[6] Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes[7] 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands[8] Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder[9] Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists[10]
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves