139009
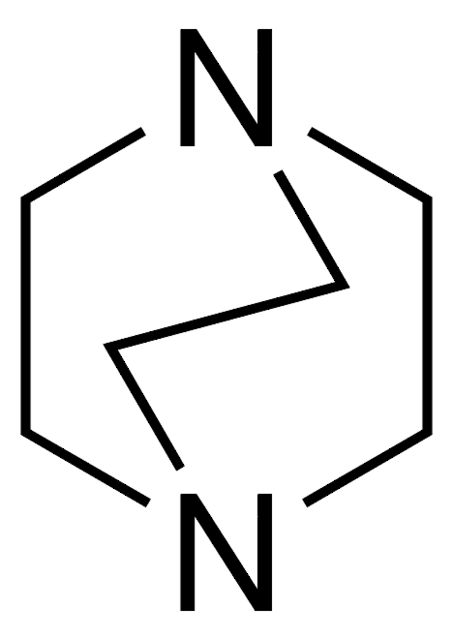
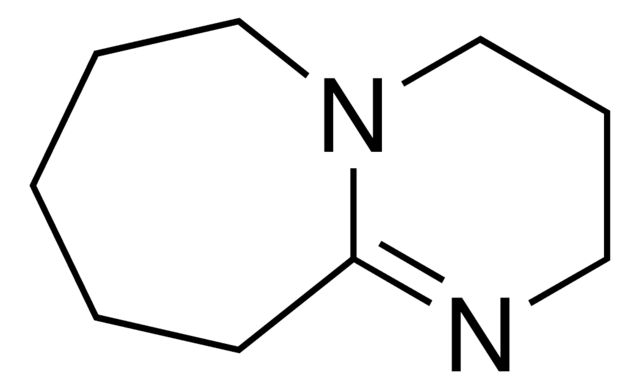
1,8-Diazabicyclo[5.4.0]undec-7-ene
98%
Manufacturer: Sigma Aldrich
Synonym(S): 2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-a]azepine, DBU
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 2 X 2 G | 139009-2-X-2-G | In Stock | ₹ 1,431.90 |
| 25 G | 139009-25-G | In Stock | ₹ 3,085.80 |
| 100 G | 139009-100-G | In Stock | ₹ 6,848.70 |
| 500 G | 139009-500-G | In Stock | ₹ 22,444.20 |
| 2.5 KG | 139009-2.5-KG | In Stock | ₹ 95,948.40 |
139009 - 2 X 2 G
In Stock
Quantity
1
Base Price: ₹ 1,431.90
GST (18%): ₹ 257.742
Total Price: ₹ 1,689.642
vapor pressure
5.3 mmHg ( 37.7 °C)
Quality Level
200
Assay
98%
form
liquid
greener alternative product characteristics
CatalysisLearn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.522-1.524 (lit.)
bp
80-83 °C/0.6 mmHg (lit.)
density
1.018 g/mL at 25 °C (lit.)
greener alternative category
, Aligned
Description
- General description: 1,8-Diazabicyclo[5.4.0]undec-7-ene is a bicyclic amidine base. It is non-nucleophilic, sterically hindered, tertiary amine base in organic chemistry. It is reported to be superior to amine catalyst in Baylis-Hillman reaction.[1] It promotes the methylation reaction of phenols, indoles and benzimidazoles with dimethyl carbonate under mild conditions.[2]
- Application: 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) may be used:as catalyst for carboxylic acid esterification with dimethyl carbonate[3] in the synthesis of duocarmycin and CC-1065 analogs[1] as catalyst in aza-Michael addition[4] and Knovenegal condensation reaction[5]as base for dehalogenation of halogenated Diels-Alder adducts and the resulting activated 2,4-dienones were subjected to regio- and stereo-directed Michael additions, using Yamamoto′s reagent (CH3Cu · BF3)[6]in a new synthesis of the ABCD ring system of Camptothecin[7]
- Features and Benefits: Strong hindered amine base.
- Citation: An application review.[10]
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P234 - P273 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
WGK
WGK 2
Flash Point(F)
240.8 °F
Flash Point(C)
116 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
vapor pressure: 5.3 mmHg ( 37.7 °C)
Quality Level: 200
Assay: 98%
form: liquid
greener alternative product characteristics: CatalysisLearn more about the Principles of Green Chemistry.
sustainability: Greener Alternative Product
refractive index: n20/D 1.522-1.524 (lit.)
bp: 80-83 °C/0.6 mmHg (lit.)
density: 1.018 g/mL at 25 °C (lit.)
greener alternative category: , Aligned
vapor pressure:
5.3 mmHg ( 37.7 °C)
Quality Level:
200
Assay:
98%
form:
liquid
greener alternative product characteristics:
CatalysisLearn more about the Principles of Green Chemistry.
sustainability:
Greener Alternative Product
refractive index:
n20/D 1.522-1.524 (lit.)
bp:
80-83 °C/0.6 mmHg (lit.)
density:
1.018 g/mL at 25 °C (lit.)
greener alternative category:
, Aligned