AI46422
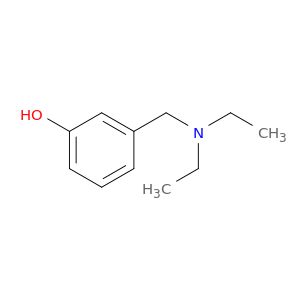
27958-96-9 | 3-[(Diethylamino)methyl]-phenol
Manufacturer: A2B Chem
The price for this product is unavailable. Please request a quote
CatalogNumber
AI46422
ChemicalName
3-[(Diethylamino)methyl]-phenol
CasNumber
27958-96-9
MolecularFormula
C11H17NO
MolecularWeight
179.2588
MdlNumber
MFCD24244267
Smiles
CCN(Cc1cccc(c1)O)CC
Complexity
134
Covalently-bondedUnitCount
1
HeavyAtomCount
13
HydrogenBondAcceptorCount
2
HydrogenBondDonorCount
1
RotatableBondCount
4
Xlogp3
1.9
Compare Similar Items
Show Difference
CatalogNumber: AI46422
ChemicalName: 3-[(Diethylamino)methyl]-phenol
CasNumber: 27958-96-9
MolecularFormula: C11H17NO
MolecularWeight: 179.2588
MdlNumber: MFCD24244267
Smiles: CCN(Cc1cccc(c1)O)CC
Complexity: 134
Covalently-bondedUnitCount: 1
HeavyAtomCount: 13
HydrogenBondAcceptorCount: 2
HydrogenBondDonorCount: 1
RotatableBondCount: 4
Xlogp3: 1.9
CatalogNumber:
AI46422
ChemicalName:
3-[(Diethylamino)methyl]-phenol
CasNumber:
27958-96-9
MolecularFormula:
C11H17NO
MolecularWeight:
179.2588
MdlNumber:
MFCD24244267
Smiles:
CCN(Cc1cccc(c1)O)CC
Complexity:
134
Covalently-bondedUnitCount:
1
HeavyAtomCount:
13
HydrogenBondAcceptorCount:
2
HydrogenBondDonorCount:
1
RotatableBondCount:
4
Xlogp3:
1.9
CatalogNumber: __
ChemicalName: __
CasNumber: __
MolecularFormula: __
MolecularWeight: __
MdlNumber: __
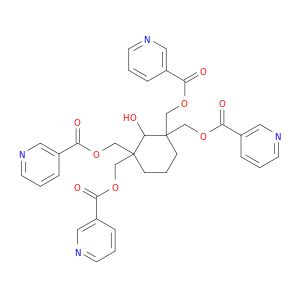
Smiles: OC1C(CCCC1(COC(=O)c1cccnc1)COC(=O)c1cccnc1)(COC(=O)c1cccnc1)COC(=O)c1cccnc1
Complexity: __
Covalently-bondedUnitCount: __
HeavyAtomCount: __
HydrogenBondAcceptorCount: __
HydrogenBondDonorCount: __
RotatableBondCount: __
Xlogp3: __
CatalogNumber:
__
ChemicalName:
__
CasNumber:
__
MolecularFormula:
__
MolecularWeight:
__
MdlNumber:
__
Smiles:
OC1C(CCCC1(COC(=O)c1cccnc1)COC(=O)c1cccnc1)(COC(=O)c1cccnc1)COC(=O)c1cccnc1
Complexity:
__
Covalently-bondedUnitCount:
__
HeavyAtomCount:
__
HydrogenBondAcceptorCount:
__
HydrogenBondDonorCount:
__
RotatableBondCount:
__
Xlogp3:
__
CatalogNumber: AI46425
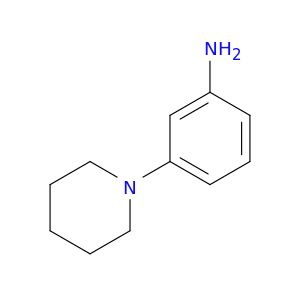
ChemicalName: 3-Piperidin-1-ylaniline
CasNumber: 27969-75-1
MolecularFormula: C11H16N2
MolecularWeight: 176.2581
MdlNumber: MFCD00234760
Smiles: Nc1cccc(c1)N1CCCCC1
Complexity: 152
Covalently-bondedUnitCount: 1
HeavyAtomCount: 13
HydrogenBondAcceptorCount: 2
HydrogenBondDonorCount: 1
RotatableBondCount: 1
Xlogp3: 2.3
CatalogNumber:
AI46425
ChemicalName:
3-Piperidin-1-ylaniline
CasNumber:
27969-75-1
MolecularFormula:
C11H16N2
MolecularWeight:
176.2581
MdlNumber:
MFCD00234760
Smiles:
Nc1cccc(c1)N1CCCCC1
Complexity:
152
Covalently-bondedUnitCount:
1
HeavyAtomCount:
13
HydrogenBondAcceptorCount:
2
HydrogenBondDonorCount:
1
RotatableBondCount:
1
Xlogp3:
2.3