22-321-135
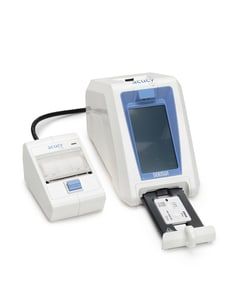
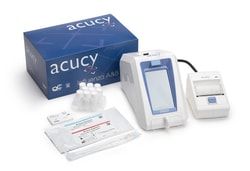
SEKISUI Diagnostics™ Acucy™ Reader System
Manufacturer: Fischer Scientific
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| Pack of 50 | 22-321-135-Pack-of-50 | In Stock | ₹ 62,300.00 |
22-321-135 - Pack of 50
In Stock
Quantity
1
Base Price: ₹ 62,300.00
GST (18%): ₹ 11,214.00
Total Price: ₹ 73,514.00
Type
In-Vitro Diagnostic Test Kit
Description
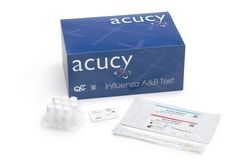
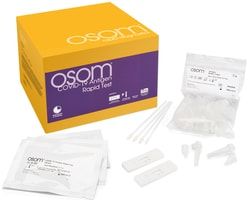
Flu A: 96.4%; Flu B: 82.3%
Detects
Influenza A, Influenza B
Regulatory Status
Clia-waived
Sample Type
Nasal Swab or NP Swab
For Use With (Application)
Nasal Swab Testing for Influenza A&B
Format
Cassette
Includes
1 Acucy™ System with all the required components and 2 Acucy™ Influenza A&B Kits
Description
- Let the Acucy™ Reader be your eyes in the lab
- This diagnostic reader uses a reflectance-based measurement method to evaluate the line signal intensities on the test strip placed inside the tray, and a specific algorithm determines the presence or absence of the target analyte
- The result from the test is displayed on the LCD screen as positive, negative, or invalid
- Easy-to-Use Scan the 2-D assay-specific barcode on the cassette leveraging the integrated scanner Place the cassette within the sample tray Place the correct number of drops of solution into the well on the test cassette and close drawer - reader will analyze the results at the appropriate time (when using walk away mode) Results are displayed on the touchscreen LCD More Flexibility for Your Busy Lab Run individual tests, or read tests in batches Walk Away Mode - no need to monitor and keep time
- Test cassette is inserted into the reader, the sample is added to the cassette well and the development and timing of the test occur within the reader, with the reader capturing the result when time is up
- Read Now Mode - run and batch multiple patient samples simultaneously
- Test cassette development time is tracked by the operator outside of the reader, then the cassettes are inserted into the reader
- Monitoring Mode - get positive results faster
- While in walk away mode, the monitoring mode can be set to read the cassette every minute of the development time
- If a positive result is detected, it will display POS (+) and then will continue to run the test until the assay-specific time limit has been reached
- Results Delivered the Way You Need Screen - test result displayed on the LCD Printed - results can be printed on the Acucy™ printer CSV Data File - each result can be transmitted to a comma-separated values (CSV) file allowing for the import of data to a computer or other external device The Acucy™ System is made up of the Acucy reader, printer, exclusive USB memory drive, password card, and calibration device
- Use only Acucy tests with the Acucy Reader
- Intended for professional and laboratory use only.
Compare Similar Items
Show Difference
Type: In-Vitro Diagnostic Test Kit
Description: Flu A: 96.4%; Flu B: 82.3%
Detects: Influenza A, Influenza B
Regulatory Status: Clia-waived
Sample Type: Nasal Swab or NP Swab
For Use With (Application): Nasal Swab Testing for Influenza A&B
Format: Cassette
Includes: 1 Acucy™ System with all the required components and 2 Acucy™ Influenza A&B Kits
Type:
In-Vitro Diagnostic Test Kit
Description:
Flu A: 96.4%; Flu B: 82.3%
Detects:
Influenza A, Influenza B
Regulatory Status:
Clia-waived
Sample Type:
Nasal Swab or NP Swab
For Use With (Application):
Nasal Swab Testing for Influenza A&B
Format:
Cassette
Includes:
1 Acucy™ System with all the required components and 2 Acucy™ Influenza A&B Kits