23-043-153
Quidel Sofia™ 2 SARS Antigen Fluorescent Immunoassay (FIA)
Manufacturer: Fischer Scientific
The price for this product is unavailable. Please request a quote
Control Sets
Positiveandnegative,includedinkit
Clia Complexity
Waived
For Use With (Equipment)
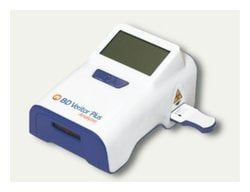
For use with Quidel Sofia 2 Analyzer, Cat. No. 20299.
Quantity
25 Tests/Pk. or 300 Tests/Cs.
Description
Test Kit
Sample Type
Nasopharyngeal
Includes
Cassettes, Reagent tubes, Reagent solution, Swabs, Pipettes (sufficient for 25 tests); Control swabs 1-pos 1-neg
Test Time
15 min.
Description
- Rapid results in 15 minutes to support efficient dispositioning of patients Objective, accurate results without cross-reactivity to seasonal coronaviruses Dual work modes adjust to volume fluctuations and allows for significant throughput and batching of samples in READ NOW Mode Seamless test workflow follows a similar format to CLIA-waived Sofia and Sofia 2 assays Virena provides automated tracking, data capture, government reporting, and exclusive disease mapping
- Fluorescent technology with automated read eliminates the subjectivity of a visual result All necessary components included in kit, ready for use for swab procedure Self-contained Test Cassette that is clean, easy to use and dispose of Note: this test kit requires an analyzer
- (See Quidel Sofia 2 Analyzer, Cat
- No
- 20299) Note: CLIA Complexity is waived
- fda.gov: The settings in which an EUA-authorized test may be used are described in the Letter of Authorization
- As discussed in the Guidance for Industry and Other Stakeholders: Emergency Use Authorization of Medical Products and Related Authorities, when the FDA authorizes tests for use at the point of care (including SARS-CoV-2 point of care test systems) under an EUA, such tests are deemed to be CLIA waived tests
- Accordingly, for the duration of the emergency declaration, such tests can be performed in a patient care setting that is qualified to have the test performed there as a result of operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Compare Similar Items
Show Difference
Control Sets: Positiveandnegative,includedinkit
Clia Complexity: Waived
For Use With (Equipment): For use with Quidel Sofia 2 Analyzer, Cat. No. 20299.
Quantity: 25 Tests/Pk. or 300 Tests/Cs.
Description: Test Kit
Sample Type: Nasopharyngeal
Includes: Cassettes, Reagent tubes, Reagent solution, Swabs, Pipettes (sufficient for 25 tests); Control swabs 1-pos 1-neg
Test Time: 15 min.
Control Sets:
Positiveandnegative,includedinkit
Clia Complexity:
Waived
For Use With (Equipment):
For use with Quidel Sofia 2 Analyzer, Cat. No. 20299.
Quantity:
25 Tests/Pk. or 300 Tests/Cs.
Description:
Test Kit
Sample Type:
Nasopharyngeal
Includes:
Cassettes, Reagent tubes, Reagent solution, Swabs, Pipettes (sufficient for 25 tests); Control swabs 1-pos 1-neg
Test Time:
15 min.