B445011
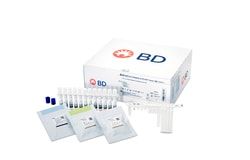
BD SARS-CoV-2/Flu for BD MAX™ System1
Manufacturer: BD
The price for this product is unavailable. Please request a quote
Detectable Analytes
SARS-CoV-2, Influenza A/B Nucleic Acids
For Use With (Application)
Simultaneous Qualitative Detection and Differentiation of Nucleic Acid From SARS-CoV-2, Influenza A and/or Influenza B
Includes
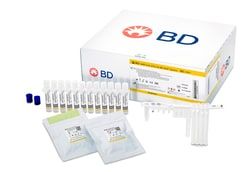
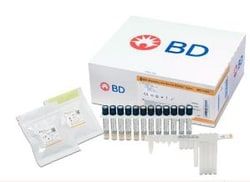
Master Mix Tubes (24), Extraction Tubes (24), Unitized Reagent Strips (24), Respiratory Sample Buffer Tubes (24), and Septum Caps (25)
Sample Type
Nasopharyngeal and Anterior Nasal Swabs
For Use With (Equipment)
BD MAX™ Systems
Quantity
24 Tests
Description
- Automated, multiplexed, real-time RT-PCR test intended for the simultaneous qualitative detection and differentiation of nucleic acid from SARS-CoV-2, influenza A and/or influenza B Nasopharyngeal and anterior nasal swabs are collected from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar Intended for use in simultaneous detection and differentiation of SARS-CoV-2, influenza A, and/or influenza B nucleic acid in clinical specimens, and is not intended to detect influenza C SARS-CoV-2, influenza A, and/or influenza B RNA is generally detectable in upper respiratory samples during the acute phase of infection Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C
- §263a, that meet the requirements to perform moderate or high complexity tests For use by qualified clinical laboratory personnel specifically instructed and trained on the use of the BD MAX™ System and in vitro diagnostic procedures Only for use under the Food and Drug Administration’s Emergency Use Authorization
Compare Similar Items
Show Difference
Detectable Analytes: SARS-CoV-2, Influenza A/B Nucleic Acids
For Use With (Application): Simultaneous Qualitative Detection and Differentiation of Nucleic Acid From SARS-CoV-2, Influenza A and/or Influenza B
Includes: Master Mix Tubes (24), Extraction Tubes (24), Unitized Reagent Strips (24), Respiratory Sample Buffer Tubes (24), and Septum Caps (25)
Sample Type: Nasopharyngeal and Anterior Nasal Swabs
For Use With (Equipment): BD MAX™ Systems
Quantity: 24 Tests
Detectable Analytes:
SARS-CoV-2, Influenza A/B Nucleic Acids
For Use With (Application):
Simultaneous Qualitative Detection and Differentiation of Nucleic Acid From SARS-CoV-2, Influenza A and/or Influenza B
Includes:
Master Mix Tubes (24), Extraction Tubes (24), Unitized Reagent Strips (24), Respiratory Sample Buffer Tubes (24), and Septum Caps (25)
Sample Type:
Nasopharyngeal and Anterior Nasal Swabs
For Use With (Equipment):
BD MAX™ Systems
Quantity:
24 Tests