S90741
Innovating Science™ AP Chemistry Kits:Electrochemical Cells
Manufacturer: Innovating Science™
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| Each of 1 | S90741-Each-of-1 | In Stock | ₹ 4,895.00 |
S90741 - Each of 1
In Stock
Quantity
1
Base Price: ₹ 4,895.00
GST (18%): ₹ 881.10
Total Price: ₹ 5,776.10
Certifications/Compliance
49 CFR 173.4
Color
White
Age
13 to 20
Includes
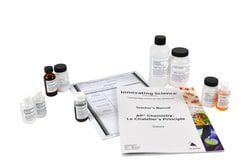
2 x 500mL Cupric Sulfate, 0.5M solution, 1 x 25mL Bromothymol Blue, 0.04% solution, 8 x 500mL Sodium Sulfate, 1M solution, Copper Metal strips, Magnesium Metal strips 5 in., dialysis tubing strips 6 in., teacher's guide and student study guide copymasters
Dimensions (L x W x H)
10.25 x 10.25 x 8.5 in.
Product Type
Electrochemical Cells Chemistry Kit
Grade
7 to 14
For Use With (Application)
To study electrolysis of water produces hydrogen and oxygen
Quantity
1
Class Size
15 Lab Groups
Related Products
Description
- The tendency of oxidation-reduction reactions is to proceed to an equilibrium state
- In electrochemical cells, these reactions provide another way for us to express the driving force in chemical reactions
- When reagents that accept or donate electrons are arranged so that the electrons can enter or leave the reaction through a metallic conductor, an electrochemical cell is established
- A half-cell contains a metal in contact with a solution of its salt
- Each metal will develop a different electrical potential based on its electron configuration
- The standard reduction potential listed in various references is the voltage that a half-cell develops when combined with a hydrogen half-cell
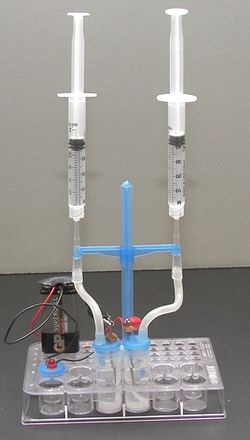
- First, construct a simple chemical battery and determine from the standard reduction potentials what the output of the battery will be (if a voltmeter is available the actual and theoretical voltages can be compared)
- Second, construct an electrolysis cell and demonstrate how hydrogen and oxygen can be produced from the electrolysis of water
- Electrolysis cell is the reverse of a battery where a voltage higher than the reversible electromotive force of the cell is applied to the electrodes In a cell containing water and an electrolyte, the oxidation reaction occurring at the anode produces oxygen gas while the reduction occurring at the cathode produces hydrogen gas By the addition of a pH indicator, the oxidation-reduction reactions can be monitored visually Gases produced are collected in inverted test tubes and the volumes of hydrogen and oxygen are compared
Compare Similar Items
Show Difference
Certifications/Compliance: 49 CFR 173.4
Color: White
Age: 13 to 20
Includes: 2 x 500mL Cupric Sulfate, 0.5M solution, 1 x 25mL Bromothymol Blue, 0.04% solution, 8 x 500mL Sodium Sulfate, 1M solution, Copper Metal strips, Magnesium Metal strips 5 in., dialysis tubing strips 6 in., teacher's guide and student study guide copymasters
Dimensions (L x W x H): 10.25 x 10.25 x 8.5 in.
Product Type: Electrochemical Cells Chemistry Kit
Grade: 7 to 14
For Use With (Application): To study electrolysis of water produces hydrogen and oxygen
Quantity: 1
Class Size: 15 Lab Groups
Certifications/Compliance:
49 CFR 173.4
Color:
White
Age:
13 to 20
Includes:
2 x 500mL Cupric Sulfate, 0.5M solution, 1 x 25mL Bromothymol Blue, 0.04% solution, 8 x 500mL Sodium Sulfate, 1M solution, Copper Metal strips, Magnesium Metal strips 5 in., dialysis tubing strips 6 in., teacher's guide and student study guide copymasters
Dimensions (L x W x H):
10.25 x 10.25 x 8.5 in.
Product Type:
Electrochemical Cells Chemistry Kit
Grade:
7 to 14
For Use With (Application):
To study electrolysis of water produces hydrogen and oxygen
Quantity:
1
Class Size:
15 Lab Groups
Certifications/Compliance: 49 CFR 173.4
Color: White
Age: 13 to 20
Includes: 4 x 500ml 0.005m edta solution, 2 x 200ml buffer solution, 2 x 15ml ebt solution (eriochrome black, 0.1%), teacher's guide and student study guide copymasters
Dimensions (L x W x H): 7.25 x 7.25 x 8.375 in.
Product Type: Water Hardness Chemistry Kit
Grade: 7 to 14
For Use With (Application): Used to signal the presence of ions in the water sample
Quantity: 1
Class Size: 15 Lab Groups
Certifications/Compliance:
49 CFR 173.4
Color:
White
Age:
13 to 20
Includes:
4 x 500ml 0.005m edta solution, 2 x 200ml buffer solution, 2 x 15ml ebt solution (eriochrome black, 0.1%), teacher's guide and student study guide copymasters
Dimensions (L x W x H):
7.25 x 7.25 x 8.375 in.
Product Type:
Water Hardness Chemistry Kit
Grade:
7 to 14
For Use With (Application):
Used to signal the presence of ions in the water sample
Quantity:
1
Class Size:
15 Lab Groups
Certifications/Compliance: __
Color: __
Age: __
Includes: Thermochemistry & Hess Law, Activity Series, Mole Ratio Reactants, Le Chatelier's Principle, Ester Formation, Acid-Base, Reaction Kinetics, Dissociation of Weak Acids, Qualitative Analysis, Beer-Lambert Law, Stoichiometry, Equilibrium Constant, Oxidation-Reduction, Freezing Point Depression, Vapor Pressure, Electrochemical Cells, Thin-Layer Chromatography, Molecular Mass of a Liquid, Thermochromism, Water Hardness, Synthesis of Aspirin, Grignard Method
Dimensions (L x W x H): __
Product Type: Complete Set Chemistry Kit
Grade: __
For Use With (Application): For lab activities
Quantity: __
Class Size: 15 Lab Groups
Certifications/Compliance:
__
Color:
__
Age:
__
Includes:
Thermochemistry & Hess Law, Activity Series, Mole Ratio Reactants, Le Chatelier's Principle, Ester Formation, Acid-Base, Reaction Kinetics, Dissociation of Weak Acids, Qualitative Analysis, Beer-Lambert Law, Stoichiometry, Equilibrium Constant, Oxidation-Reduction, Freezing Point Depression, Vapor Pressure, Electrochemical Cells, Thin-Layer Chromatography, Molecular Mass of a Liquid, Thermochromism, Water Hardness, Synthesis of Aspirin, Grignard Method
Dimensions (L x W x H):
__
Product Type:
Complete Set Chemistry Kit
Grade:
__
For Use With (Application):
For lab activities
Quantity:
__
Class Size:
15 Lab Groups
Certifications/Compliance: __
Color: __
Age: __
Includes: __
Dimensions (L x W x H): __
Product Type: Human Skeleton Chart
Grade: __
For Use With (Application): An easy study of the bones of the body, great addition to any classroom or doctor's office, useful tool for patient education
Quantity: __
Class Size: __
Certifications/Compliance:
__
Color:
__
Age:
__
Includes:
__
Dimensions (L x W x H):
__
Product Type:
Human Skeleton Chart
Grade:
__
For Use With (Application):
An easy study of the bones of the body, great addition to any classroom or doctor's office, useful tool for patient education
Quantity:
__
Class Size:
__