3563410
Deoxyribonuclease I from Bovine pancreas, MP Biomedicals™
Deoxyribonuclease I from Bovine pancreas 50,000 Dornase u/mg solid, White powder
Manufacturer: Fischer Scientific
The price for this product is unavailable. Please request a quote
Color
White
Description
- Deoxyribonuclease from beef pancreas, DNase I, was first crystallized by Kunitz
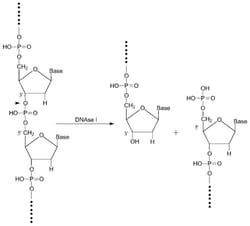
- It is an endonuclease which splits phosphodiester linkages, preferentially adjacent to a pyrimidine nucleotide yielding 5'-phosphate terminated polynucleotides with a free hydroxyl group on position 3'
- The average chain of limit digest is a tetranucleotide
- DNase I acts upon single chain DNA, and upon double-stranded DNA and chromatin
- In the latter case, although histones restrict susceptibility to nuclease action, over a period of time nearly all chromatin DNA is acted upon
- According to Mirsky and Silverman, this could result from the looseness of histone attachment to DNA
- They found that lysine-rich histones more effectively block DNase access to DNA than arginine-rich histones