8889000
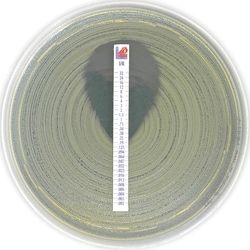
Liofilchem™ MTS™ Imipenem-relebactam I/R 0.002/4-32/4
A quantitative assay for determining the Minimum Inhibitory Concentration (MIC) of antimicrobial agents against microorganisms to indicate appropriate patient treatment and for identifying resistance patterns.
Manufacturer: Fischer Scientific
The price for this product is unavailable. Please request a quote
Antimicrobial Agents
Imipenem-relebactam
Concentration
0.002/4 - 32/4 μg/mL
Description
Liofilchem™ MTS™ Imipenem-relebactam
Product Type
MIC Test Strip
Certifications/Compliance
FDA Cleared
Content And Storage
-20°C or 2°C to 8°C until the given expiry date
Antibiotic Code
I/R
For Use With (Application)
Antimicrobial Susceptibility Testing, For determining the MIC of antimicrobial agents against microorganisms
Description
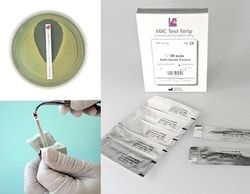
- Ready to use: MTS™ (MIC Test Strip) is a ready to use MIC device
- No preparation or dilution of antimicrobial agent is required
- The MTS™ in simply placed on an inoculated agar plate and incubated
- MIC testing for a wide range of agents and against many organisms can be done on request and at short notice
- Synergy Testing: MTS™ can be crossed for evaluating a critical assay such as the in vitro combination of antibiotics (MTS™ Synergy Applicator System: A device for standardizing the in vitro synergy testing of two antibiotics, US patent)
- Rapid and Efficient: MTS™ is easy to set up and full range of MIC testing of 6 agents on a large plate takes no more than 5 minutes
- Solid Medium: Growth of organisms on agar better mimics in vitro conditions
- It is easy to visualize hetero resistant variants in a population and to detect mixed cultures and contamination
- Inoculum Tolerant, Macro.method: Most AST methods including broth microdilution and automated systems, are done in a micro format using a relatively low inoculum concentration
- These systems do not mimic the in vitro infection site nor is the inoculum chosen large enough to reflect the heavy colonization of organisms e.g
- in endocarditis and meningitis
- Further, the accurate detection of clinically significant low level and hetero resistance may be compromised
- MTS™ is a stable gradient technique that is inoculum tolerant i.e
- changes in inoculum do not affect the MIC results for a susceptible strain
- Thus, it can be used in a macro format with heavier inoculum and a richer media to maximize the detection of low level and hetero resistance
- Easy to Handle: Each MTS™ package contains 100 strips, 30 strips or 10 strips
- The package is small and light weigh
- Required storage space is minimal
- The unopened package of MTS™ should be stored at -20°C or 2-8°C until the given expiry date
- Leftover MTS™ from an opened package (valid for 100 strip pack only, as the 10 and 30 strip packs contain individually packed strips) must be stored at 2-8°C in the airtight tube, containing desiccant, provided in the pack for no more than 7 days
- Wide MIC Range: The MIC range of Each MTS™ gradient spans across 15 dilutions fulfilling pharmacokinetic needs, varying susceptibility of many organism groups and covers different MIC breakpoints used in different countries as recommended by various reference or expert groups
- Easy to Learn: Setting up MTS™ is as easy as setting up a disc test, an established procedure familiar to all laboratories worldwide for over 50 years
- Minimal training is required for clinical microbiology personnel
- No equipment is required for MTS™ use
- Easy for New Agents: When MTS™ becomes available for new agents, these can be easily introduced into clinical routines, as MTS™ is a stand*alone single reagent not a panel product
- New agents are usually rapidly available in the MTS™ format compared to panel AST systems
- Clinically, new agents are becoming increasingly important to test and consider as alternative therapeutic options due to increasing resistance
- MIC Equivalent to Reference Values: Scientific references and the clinical testing for device approval has validated that MTS™ MIC values are substantially equivalent to reference values from the CLSI agar and broth microdilution methods
- Thus, MIC interpretive criteria from international standards can be directly applied to interpret MTS™ MIC results
- Improved MIC Precision: The precise MIC value in μg/ mL is read directly from MTS™
- MIC values from MTS™ have an inherent precision of 1/2 dilution due to the continuous gradient
- MTS™ provides an exact on scale MIC value that is not an “in*between” two*fold dilution value
- Flexible Media Choice: MTS™ can be used with several AST media provided that the medium supports growth of the organism and is defined and standardized for susceptibility testing
- In fact, MTS™ can be used as a quality control tool for checking different AST media, brand to brand and batch to batch
- Robust Technique: As a stable gradient technique, MTS™ is relatively tolerant to variations that can be daily encountered in the laboratory e.g
- inoculum variations and technician performance and can still generate good intra and inter laboratory reproducibility
- Quantitative Value: MTS™ generates quantitative MIC values that are needed to guide therapy selection for the individual patient * choice, dose and regimen
- Fastidious and Slow Growers: Unlike many automated systems, MTS™ can be used for fastidious organisms e.g
- anaerobes, and other slow growing organisms and incubation can vary from 1 to 5 days
- Direct Specimen Testing: Due to the gradient and method flexibility, MTS™ can be used to directly test positive blood cultures, CSF and sputum from cystic fibrosis patients and bronchial aspirates from VAP patients
- The extra day saved by testing the specimen directly can have significant impact in therapy management, patient care and hospital cost savings.
Compare Similar Items
Show Difference
Antimicrobial Agents: Imipenem-relebactam
Concentration: 0.002/4 - 32/4 μg/mL
Description: Liofilchem™ MTS™ Imipenem-relebactam
Product Type: MIC Test Strip
Certifications/Compliance: FDA Cleared
Content And Storage: -20°C or 2°C to 8°C until the given expiry date
Antibiotic Code: I/R
For Use With (Application): Antimicrobial Susceptibility Testing, For determining the MIC of antimicrobial agents against microorganisms
Antimicrobial Agents:
Imipenem-relebactam
Concentration:
0.002/4 - 32/4 μg/mL
Description:
Liofilchem™ MTS™ Imipenem-relebactam
Product Type:
MIC Test Strip
Certifications/Compliance:
FDA Cleared
Content And Storage:
-20°C or 2°C to 8°C until the given expiry date
Antibiotic Code:
I/R
For Use With (Application):
Antimicrobial Susceptibility Testing, For determining the MIC of antimicrobial agents against microorganisms
Antimicrobial Agents: __
Concentration: __
Description: __
Product Type: __
Certifications/Compliance: CAN/CSA C22.2 61010-1; UL 61010-1; AC adapter: CAN/CSA C22 60950-1; UL 60950-1; Canada ICES-003 Class B; FCC Part 15 Class B; T32MC indicator: cCSAus; AC adapter: cULus
Content And Storage: __
Antibiotic Code: __
For Use With (Application): __
Antimicrobial Agents:
__
Concentration:
__
Description:
__
Product Type:
__
Certifications/Compliance:
CAN/CSA C22.2 61010-1; UL 61010-1; AC adapter: CAN/CSA C22 60950-1; UL 60950-1; Canada ICES-003 Class B; FCC Part 15 Class B; T32MC indicator: cCSAus; AC adapter: cULus
Content And Storage:
__
Antibiotic Code:
__
For Use With (Application):
__