1.09136
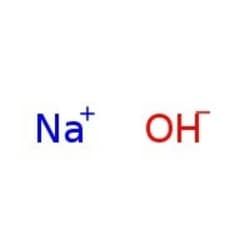
Sodium hydroxide solution
c(NaOH) = 2 mol/l (2 N), Titripur®
Manufacturer: Supelco
CAS Number: 1310-73-2
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 L | 1.09136-1-L | In Stock | ₹ 3,390.00 |
| 25 L | 1.09136-25-L | In Stock | ₹ 27,830.01 |
1.09136 - 1 L
In Stock
Quantity
1
Base Price: ₹ 3,390.00
GST (18%): ₹ 610.20
Total Price: ₹ 4,000.20
vapor pressure
3 mmHg ( 37 °C)
Quality Level
200
product line
Titripur®
form
liquid
quality
Analyzed in our ISO 17025 accredited QC lab
reaction suitability
reaction type: Acid-base reactions
concentration
2 M
technique(s)
titration: suitable
pH
13.8 (20 °C in H2O)
density
1.09 g/cm3 at 20 °C
Other Options
...
Description
- Application: Transitory alkali exposure on meibomian gland orifices induces meibomian gland dysfunction.: This research utilizes sodium hydroxide solution to create a model of meibomian gland dysfunction by transiently exposing gland orifices to alkali, providing insights into the pathophysiology of dry eye diseases and potential therapeutic approaches (Bu J et al., 2023). Radionuclides′ Recovery from Seawater Using FIC and FIC A Sorbents.: This study utilizes sodium hydroxide solution in the processing of innovative sorbents designed for the efficient recovery of radionuclides from seawater, highlighting its role in environmental protection and nuclear waste management (Bezhin NA et al., 2023). Synthesis of Chitosan-Silver Nanocomposite and Its Evaluation as an Antibacterial Coating for Mobile Phone Glass Protectors.: Demonstrating the application of sodium hydroxide solution in the synthesis of chitosan-silver nanocomposites, this study explores their potential as antibacterial coatings for mobile phone protectors, contributing to public health by reducing pathogen transmission on high-touch surfaces (Canama GJC et al., 2023).
- Features and Benefits: Accreditation:This volumetric solution is analyzed by our calibration laboratory D-K-15185-01-00 which is accredited according to DIN EN ISO/IEC 17025 for analysis of amount-of-substance concentrations in volumetric solutions by DAkkS (Deutsche Akkreditierungsstelle - German National Accreditation Body). The accreditation certificate can be found at www.sigmaaldrich.com/ISO17025.
- Packaging: SKU-pack size numbers ending with a "3" are SmartChemicals with an RFID tag on the label for seamless data transfer to the instrument, e.g. xxxxxx1003 or xxxxxxx 4003. More information on www.sigmaaldrich.com/SmartChemicals
- Analysis Note: Form liquidAmount-of-substance concentration 1.990 - 2.010 mol/LMeasurement uncertainty ± 0.006 mol/LTraceability NIST SRMThe concentration is determined by volumetric titration and refers to 20°C.The amount-of-substance concentration of this volumetric solution is traceable to a primary standard reference material (SRM) from the National Institute of Standards and Technology, Gaithersburg, USA (NIST SRM 84 potassium hydrogen phthalate) by means of volumetric standard potassium hydrogen phthalate (article number 1.02400), certified reference material according to ISO 17034, analyzed by our accredited calibration laboratory of Merck KGaA, Darmstadt, Germany according to DIN EN ISO/IEC 17025. The uncertainty is expressed as expanded measurement uncertainty with a coverage factor k=2 covering a confidence level of 95%.Note: The titer is a correction factor to correct for variations of the volumetric solution, the titration equipment, the temperature and other laboratory conditions. For correct titration results it is recommended to determine a titer with the laboratory specific equipment and under laboratory specific conditions directly after opening a new bottle and at regular time intervals.
- Legal Information: Titripur is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P234 - P280 - P303 + P361 + P353 - P304 + P340 + P310 - P305 + P351 + P338 - P363
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
vapor pressure: 3 mmHg ( 37 °C)
Quality Level: 200
product line: Titripur®
form: liquid
quality: Analyzed in our ISO 17025 accredited QC lab
reaction suitability: reaction type: Acid-base reactions
concentration: 2 M
technique(s): titration: suitable
pH: 13.8 (20 °C in H2O)
density: 1.09 g/cm3 at 20 °C
vapor pressure:
3 mmHg ( 37 °C)
Quality Level:
200
product line:
Titripur®
form:
liquid
quality:
Analyzed in our ISO 17025 accredited QC lab
reaction suitability:
reaction type: Acid-base reactions
concentration:
2 M
technique(s):
titration: suitable
pH:
13.8 (20 °C in H2O)
density:
1.09 g/cm3 at 20 °C