30020
D-Cycloserine
Manufacturer: Sigma Aldrich
CAS Number: 68-41-7
Synonym(S): R-4-Amino-3-isoxazolidinone, (R)-4-Amino-3-isoxazolidone, 4-Amino-3-isoxazolidinone
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 30020-1-G | In Stock | ₹ 6,743.98 |
| 5 G | 30020-5-G | In Stock | ₹ 28,025.93 |
| 25 G | 30020-25-G | In Stock | ₹ 91,666.10 |
30020 - 1 G
In Stock
Quantity
1
Base Price: ₹ 6,743.98
GST (18%): ₹ 1,213.916
Total Price: ₹ 7,957.896
biological source
synthetic
Quality Level
100
form
powder
potency
≥900 μg per mg
color
white to off-white
mp
147 °C (dec.) (lit.)
antibiotic activity spectrum
Gram-negative bacteriaGram-positive bacteriamycobacteria
Mode of action
cell wall synthesis | interferes
storage temp.
−20°C
SMILES string
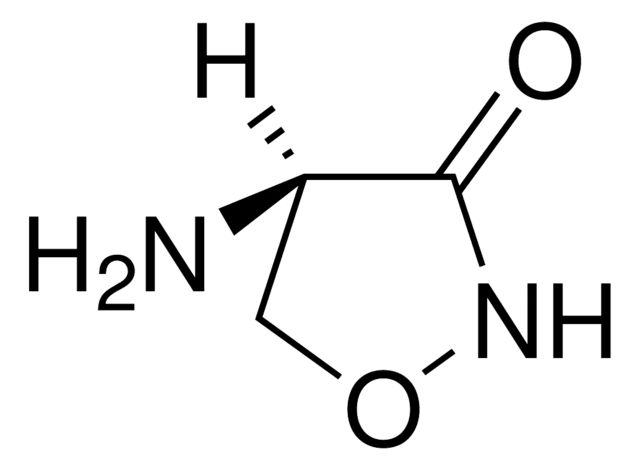
N[C@@H]1CONC1=O
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Sigma Aldrich Fine Chemicals Biosciences D-Cycloserine | 68-41-7 | MFCD00005353 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 10,291.96 | |
 | Medchemexpress LLC HY-B0030 500mg Medchemexpress, D-Cycloserine CAS:68-41-7 Purity:>98% | Medchemexpress LLC | ₹ 6,541.50 | |
 | Chem-Impex International, Inc. D-Cycloserine | 68-41-7 | MFCD00005353 | 5G | Chem-Impex International, Inc. | ₹ 4,564.81 | |
 | Chem-Impex International, Inc. D-Cycloserine, synthetic | 68-41-7 | MFCD00005353 | 1G | Chem-Impex International, Inc. | ₹ 3,003.75 | |
 | Cycloserine | Supelco | ₹ 11,647.70 | |
 | D-Cycloserine | Sigma Aldrich | ₹ 7,739.88 - ₹ 27,246.53 | |
 | D-Cycloserine | Sigma Aldrich | ₹ 7,739.88 - ₹ 27,246.53 | |
 | D-Cycloserine | Sigma Aldrich | ₹ 6,743.98 - ₹ 91,666.10 | |
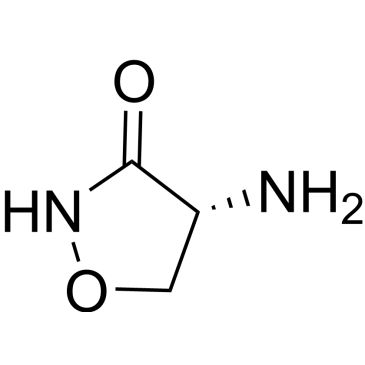 | D-Cycloserine | ChemScene | ₹ 445.00 - ₹ 42,987.00 | |
 | 68-41-7 | (4R)-4-amino-1,2-oxazolidin-3-one | A2B Chem | ₹ 1,424.00 - ₹ 41,474.00 |
Related Products
Description
- General description: Chemical structure: amino acid derivatives
- Application: D-Cycloserine acts as inhibitor of various enzymes.[1]
- Biochem/physiol Actions: Mode of Action: Inhibits cell wall biosynthesis (D-Ala peptide bond formation). Also prevents conversion of D-Ala to L-Ala. Bacteriostatic. Mode of Resistance: D-Ala transport interference.
- Packaging: 1g, 5g, 25g
- Other Notes: Keep container tightly closed in a dry and well-ventilated place. Store under inert gas. Air sensitive. Keep in a dry place.
SAFETY INFORMATION
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
biological source: synthetic
Quality Level: 100
form: powder
potency: ≥900 μg per mg
color: white to off-white
mp: 147 °C (dec.) (lit.)
antibiotic activity spectrum: Gram-negative bacteriaGram-positive bacteriamycobacteria
Mode of action: cell wall synthesis | interferes
storage temp.: −20°C
SMILES string: N[C@@H]1CONC1=O
biological source:
synthetic
Quality Level:
100
form:
powder
potency:
≥900 μg per mg
color:
white to off-white
mp:
147 °C (dec.) (lit.)
antibiotic activity spectrum:
Gram-negative bacteriaGram-positive bacteriamycobacteria
Mode of action:
cell wall synthesis | interferes
storage temp.:
−20°C
SMILES string:
N[C@@H]1CONC1=O
biological source: __
Quality Level: 200
form: powder
potency: __
color: white to off-white
mp: __
antibiotic activity spectrum: fungi
Mode of action: enzyme | inhibits
storage temp.: 2-8°C
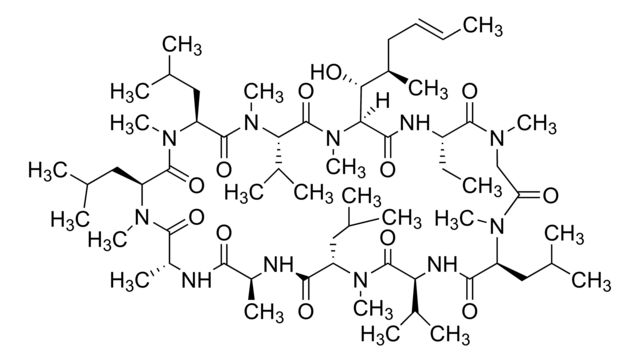
SMILES string: CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C
biological source:
__
Quality Level:
200
form:
powder
potency:
__
color:
white to off-white
mp:
__
antibiotic activity spectrum:
fungi
Mode of action:
enzyme | inhibits
storage temp.:
2-8°C
SMILES string:
CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C