773069
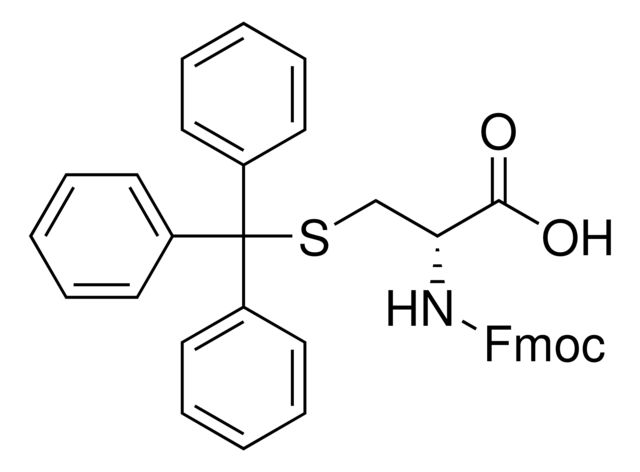
Fmoc-N-Me-Cys(Trt)-OH
97% (HPLC)
Manufacturer: Sigma Aldrich
CAS Number: 944797-51-7
Synonym(S): N-α-Fmoc-N-α-methyl-S-trityl-L-cysteine
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | 773069-1-G | In Stock | ₹ 44,414.98 |
773069 - 1 G
In Stock
Quantity
1
Base Price: ₹ 44,414.98
GST (18%): ₹ 7,994.696
Total Price: ₹ 52,409.676
Quality Level
100
Assay
97% (HPLC)
form
powder or crystals
optical activity
[α]22/D -25.0°, c = 0.5% in dichloromethane
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
mp
234-239 °C
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
InChI
1S/C38H33NO4S/c1-39(37(42)43-25-34-32-23-13-11-21-30(32)31-22-12-14-24-33(31)34)35(36(40)41)26-44-38(27-15-5-2-6-16-27,28-17-7-3-8-18-28)29-19-9-4-10-20-29/h2-24,34-35H,25-26H2,1H3,(H,40,41)/t35-/m0/s1
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | eMolecules Fmoc-N-methyl-L-cysteine(Trt) | 944797-51-7 | MFCD11973909 | 5g | eMolecules | ₹ 28,821.76 | |
 | Sigma Aldrich Fine Chemicals Biosciences Fmoc-N-Me-Cys(Trt)-OH 97% (HPLC) | 944797-51-7 | 1G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 67,632.88 | |
 | Fmoc-N-Me-Cys(Trt)-OH | Sigma Aldrich | ₹ 44,414.98 | |
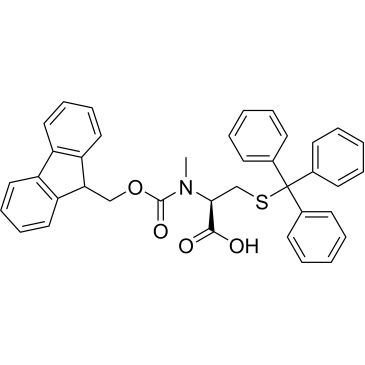 | N-(((9H-Fluoren-9-yl)methoxy)carbonyl)-N-methyl-S-trityl-L-cysteine | ChemScene | ₹ 4,539.00 - ₹ 79,922.00 | |
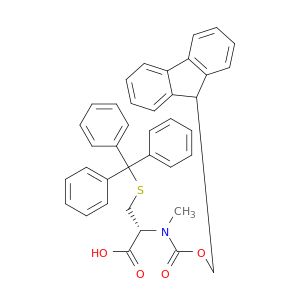 | 944797-51-7 | Fmoc-N-methyl-L-cysteine(Trt) | A2B Chem | ₹ 4,539.00 - ₹ 2,40,923.00 |
Related Products
Description
- Application: Fmoc-N-Me-Cys(Trt)-OH is a Fmoc-protected derivative of N-methyl cysteine used as a building block to prepare peptide thioesters under acidic conditions. The residue attached to the amino group of N-methylcysteine can migrate to the cysteinyl thiol group, resulting in the formation of a peptide thioester. The introduction of this Fmoc-protected derivative is best achieved using HATU as a coupling reagent in the presence of DIPEA (N, N-Diisopropylethylamine). It can also be used to prepare Fmoc-N-Me-Cys(Trt)-OAllyl intermediate for the solid-phase synthesis of dithiol Triostin A.[1]
SAFETY INFORMATION
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Compare Similar Items
Show Difference
Quality Level: 100
Assay: 97% (HPLC)
form: powder or crystals
optical activity: [α]22/D -25.0°, c = 0.5% in dichloromethane
reaction suitability: reaction type: Fmoc solid-phase peptide synthesis
mp: 234-239 °C
application(s): peptide synthesis
functional group: Fmoc
storage temp.: 2-8°C
InChI: 1S/C38H33NO4S/c1-39(37(42)43-25-34-32-23-13-11-21-30(32)31-22-12-14-24-33(31)34)35(36(40)41)26-44-38(27-15-5-2-6-16-27,28-17-7-3-8-18-28)29-19-9-4-10-20-29/h2-24,34-35H,25-26H2,1H3,(H,40,41)/t35-/m0/s1
Quality Level:
100
Assay:
97% (HPLC)
form:
powder or crystals
optical activity:
[α]22/D -25.0°, c = 0.5% in dichloromethane
reaction suitability:
reaction type: Fmoc solid-phase peptide synthesis
mp:
234-239 °C
application(s):
peptide synthesis
functional group:
Fmoc
storage temp.:
2-8°C
InChI:
1S/C38H33NO4S/c1-39(37(42)43-25-34-32-23-13-11-21-30(32)31-22-12-14-24-33(31)34)35(36(40)41)26-44-38(27-15-5-2-6-16-27,28-17-7-3-8-18-28)29-19-9-4-10-20-29/h2-24,34-35H,25-26H2,1H3,(H,40,41)/t35-/m0/s1