C7698
Cycloheximide
from microbial, ≥94% (TLC)
Manufacturer: Sigma Aldrich
CAS Number: 66-81-9
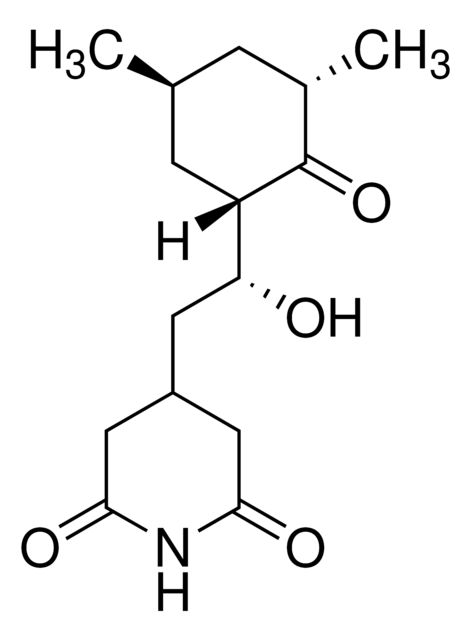
Synonym(S): 3-[2-(3,5-Dimethyl-2-oxocyclohexyl)-2-hydroxyethyl]glutarimide, Actidione, Naramycin A
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | C7698-1-G | In Stock | ₹ 10,770.88 |
| 5 G | C7698-5-G | In Stock | ₹ 25,417.10 |
C7698 - 1 G
In Stock
Quantity
1
Base Price: ₹ 10,770.88
GST (18%): ₹ 1,938.758
Total Price: ₹ 12,709.638
biological source
microbial
Quality Level
200
Assay
≥94% (TLC)
form
powder
color
white to off-white
solubility
ethanol: soluble, clear to hazy
antibiotic activity spectrum
fungiyeast
Mode of action
protein synthesis | interferes
storage temp.
2-8°C
SMILES string
[H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC2CC(=O)NC(=O)C2
Other Options
Description
- General description: Cycloheximide, also known as Actidione, is a glutarimide antibiotic commonly derived from the bacterium Streptomyces griseus. It acts as a potent inhibitor of protein biosynthesis in eukaryotic cells by disrupting the translocation step during translation, effectively blocking translational elongation. In microbiology, it plays a crucial role as a selection agent for resistant strains of yeast and fungi, proving invaluable in controlled experimental environments.In cell biology and biochemical research, Cycloheximide showcases a dual nature concerning Apoptosis Induction, inducing or inhibiting apoptosis depending on the cell type. Its rapid and reversible effects make it an ideal choice for studying cellular processes and determining protein half-life. Cycloheximide also finds extensive applications in biomedical research, where it inhibits protein synthesis in eukaryotic cells studied in vitro (outside of organisms) and its effects are rapidly reversed by simply removing it. This makes Cycloheximide a go-to choice for exploring cell biology, biomedical and biochemical research, offering precise control and versatility in experiments.
- Application: In yeast strains, cycloheximide has been used as a protein synthesis inhibitor in the cycloheximide chase experiment.[1] It has been used to inhibit translation in mammalian cells.[2][3]It has been used to suppress fungal growth.[4]
- Biochem/physiol Actions: Mode of Action: Translation inhibition in eukaryotes resulting in cell growth arrest and cell death. CHX is widely used for the selection of CHX-resistant strains of yeast and fungi, controlled inhibition of protein synthesis for detection of short-lived proteins and super-induction of protein expression, and apoptosis induction or facilitation of apoptosis induction by death receptors. Activity Spectrum: Active against yeast and fungi like Candida, Aspergillus, Saccharomyces, Penicillium
- Features and Benefits: High-quality antibiotic suitable for multiple research applicationsCommonly used in Cell Biology and Biochemical applications
- Storage and Stability: Tightly closed. Dry. Keep in a well-ventilated place. Keep locked up or in an area accessible only to qualified or authorized persons.
- Other Notes: For additional information on our range of Biochemicals, please complete this form.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Aquatic Chronic 2 - Muta. 2 - Repr. 1B
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Compare Similar Items
Show Difference
biological source: microbial
Quality Level: 200
Assay: ≥94% (TLC)
form: powder
color: white to off-white
solubility: ethanol: soluble, clear to hazy
antibiotic activity spectrum: fungiyeast
Mode of action: protein synthesis | interferes
storage temp.: 2-8°C
SMILES string: [H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC2CC(=O)NC(=O)C2
biological source:
microbial
Quality Level:
200
Assay:
≥94% (TLC)
form:
powder
color:
white to off-white
solubility:
ethanol: soluble, clear to hazy
antibiotic activity spectrum:
fungiyeast
Mode of action:
protein synthesis | interferes
storage temp.:
2-8°C
SMILES string:
[H][C@]1(C[C@@H](C)C[C@H](C)C1=O)[C@H](O)CC2CC(=O)NC(=O)C2
biological source: __
Quality Level: 200
Assay: ≥80% (Curcumin)≥94% (curcuminoid content)
form: powder
color: __
solubility: ethanol: 1 mg/mLDMSO: >11 mg/mL0.5 M NaOH: soluble (then immediately diluted in PBS)
antibiotic activity spectrum: __
Mode of action: __
storage temp.: −20°C
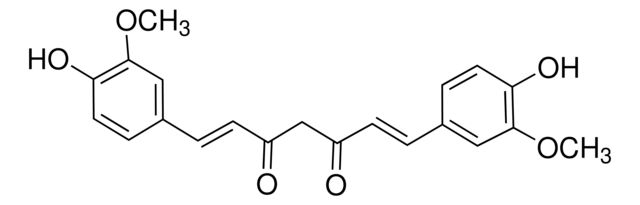
SMILES string: COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O
biological source:
__
Quality Level:
200
Assay:
≥80% (Curcumin)≥94% (curcuminoid content)
form:
powder
color:
__
solubility:
ethanol: 1 mg/mLDMSO: >11 mg/mL0.5 M NaOH: soluble (then immediately diluted in PBS)
antibiotic activity spectrum:
__
Mode of action:
__
storage temp.:
−20°C
SMILES string:
COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O