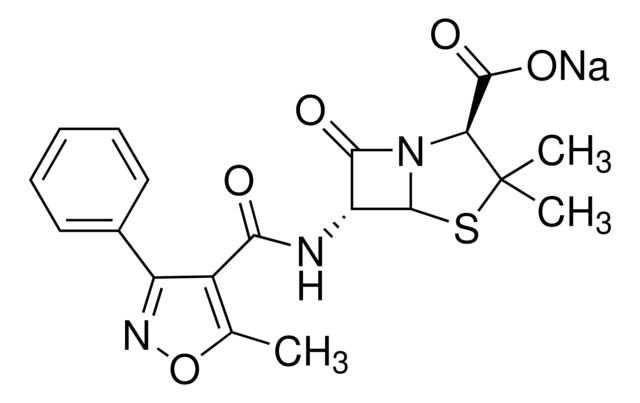
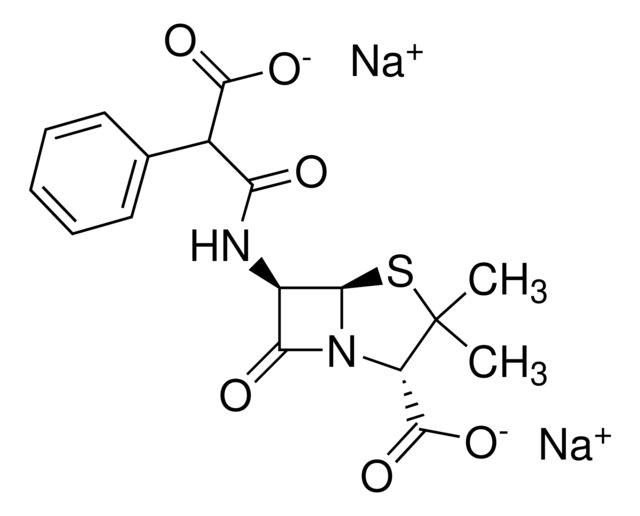
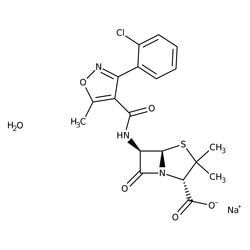
Cloxacillin sodium salt monohydrate
β-lactamase-resistant antibiotic
Manufacturer: Sigma Aldrich
CAS Number: 7081-44-9
Synonym(S): Cloxacillin, Sodium cloxacillin monohydrate
Select a Size
| Pack Size | SKU | Availability | Price |
|---|---|---|---|
| 1 G | C9393-1-G | In Stock | ₹ 6,083.65 |
| 5 G | C9393-5-G | In Stock | ₹ 16,085.95 |
C9393 - 1 G
In Stock
Quantity
1
Base Price: ₹ 6,083.65
GST (18%): ₹ 1,095.057
Total Price: ₹ 7,178.707
Quality Level
200
form
powder or crystals
antibiotic activity spectrum
Gram-positive bacteria
Mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
SMILES string
O.[Na+].Cc1onc(-c2ccccc2Cl)c1C(=O)N[C@H]3[C@H]4SC(C)(C)[C@@H](N4C3=O)C([O-])=O
InChI
1S/C19H18ClN3O5S.Na.H2O/c1-8-11(12(22-28-8)9-6-4-5-7-10(9)20)15(24)21-13-16(25)23-14(18(26)27)19(2,3)29-17(13)23;;/h4-7,13-14,17H,1-3H3,(H,21,24)(H,26,27);;1H2/q;+1;/p-1/t13-,14+,17-;;/m1../s1
InChI key
KCUWTKOTPIUBRI-VICXVTCVSA-M
Other Options
| Image | Product Name | Manufacturer | Price Range | |
|---|---|---|---|---|
 | Cloxacillin Sodium Pharmaceutical Secondary Standard, MilliporeSigma™ Supelco™ | MilliporeSigma Supelco | ₹ 20,114.00 | |
 | Sigma Aldrich Fine Chemicals Biosciences Cloxacillin sodium European Pharmacopoeia (EP) Reference Standard | 7081-44-9 | MFCD00150735 | | Sigma Aldrich Fine Chemicals Biosciences | ₹ 17,007.90 | |
 | Sigma Aldrich Fine Chemicals Biosciences Cloxacillin sodium salt monohydrate beta-lactamase-resistant antibiotic | 7081-44-9 | MFCD00150735 | 5G | Sigma Aldrich Fine Chemicals Biosciences | ₹ 28,782.60 | |
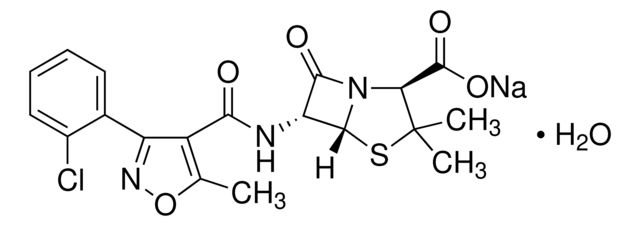 | Cloxacillin sodium salt monohydrate | Supelco | ₹ 6,137.78 | |
 | Cloxacillin Sodium | Supelco | ₹ 18,976.23 | |
 | Cloxacillin sodium | Sigma Aldrich | ₹ 14,267.35 | |
 | Cloxacillin sodium salt monohydrate | Sigma Aldrich | ₹ 6,083.65 - ₹ 16,085.95 | |
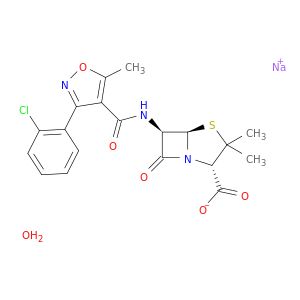 | 7081-44-9 | Cloxacillin sodium salt monohydrate | A2B Chem | ₹ 3,382.00 - ₹ 9,612.00 |
Description
- General description: Chemical structure: ß-lactam
- Application: Cloxacillin is effective against infections caused by penicillin G-resistant staphylococci.[1] It was used in studies of topical antibacterial agents for burn and crush wounds.[2]
- Biochem/physiol Actions: Cloxacillin is a semi-synthetic antibiotic and a chlorinated derivative of oxacillin. It inhibits the last stage of bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs), which results in cell lysis. Cell lysis is mediated by bacterial cell wall autolytic enzymes. Cloxacillin is often used as AmpC β-lactamase inhibitor in combination with β-lactam antibiotics.
- Other Notes: Keep container tightly closed in a dry and well-ventilated place.
SAFETY INFORMATION
Pictograms
Signal Word
Danger
Hazard Statements
Precautionary Statements
P261 - P264 - P271 - P280 - P302 + P352 - P305 + P351 + P338
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves